Supplementing to Survive
Many biomarkers decline with age, but can you supplement to compensate and recover your health?
Join the club for FREE to access the whole archive and other member benefits.
Navigating the vast and diverse supplement market can be daunting, considering the wide range of available options, each promoting distinct attributes and pricing. A very common is the existence of two brands of the same supplement, claiming similar ingredients and benefits but differing significantly in cost – one being more expensive, while the other remains more affordable.
This situation prompts us to ponder whether we should opt for the expensive option, assuming that a higher price equates to superior quality, or the cheaper alternative is viable. With this in mind, the objective of this blog is to provide valuable insights into the essential considerations when delving into the world of supplement purchasing.
When considering health products, placing paramount importance on quality is crucial, considering their direct impact on our well-being. Unlike the pharmaceutical industry, the dietary supplement sector lacks stringent regulations, resulting in limited oversight and considerable price disparities for products with seemingly identical ingredients.

This unregulated supplement industry results in self-made rules, allowing misleading claims and inconsistent nutritional labelling. For instance, 2021 research on athletes' supplement use revealed transparency issues, raising safety concerns[1]. Studies have also found some athletes testing positive for banned substances attributed their use to unexpected ingredients in dietary supplements[2].
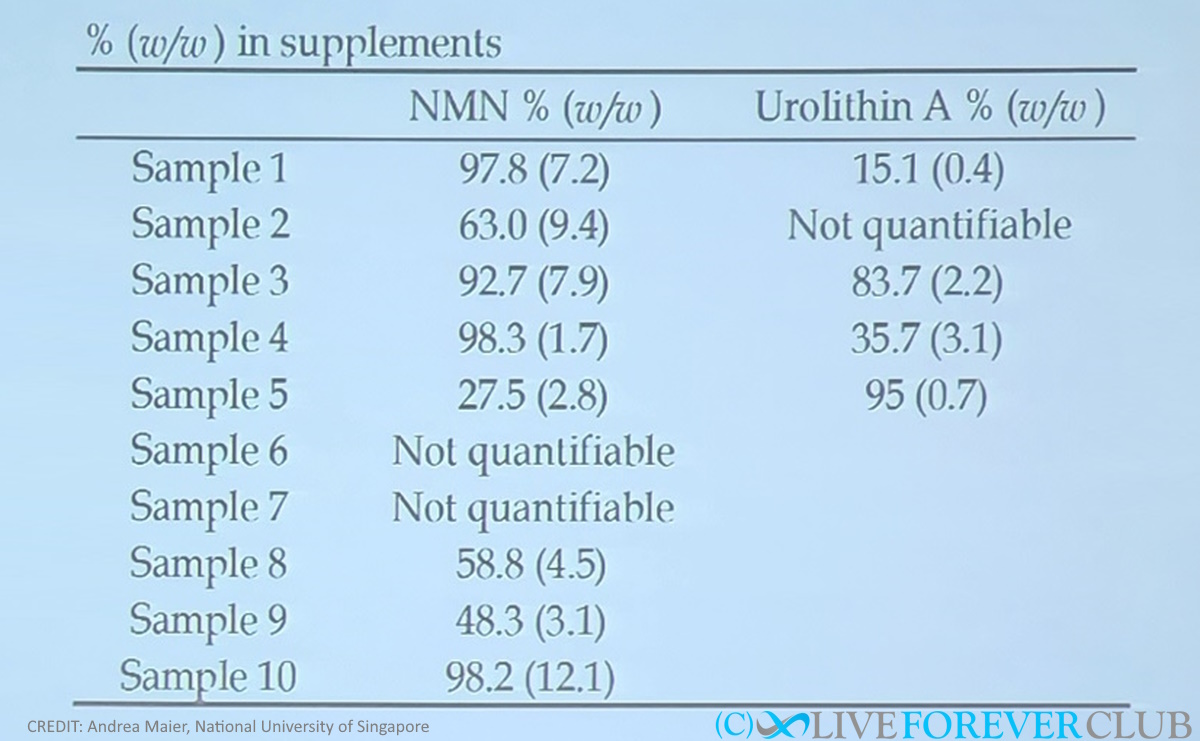
Misleading claims are not limited to the sports industry, as even common supplement ingredients such as caffeine lack adequate health claims supported by authorities and proper labelling[3]. In another example, a soon to be published study by Andrea Maier et al found the contents of NMN and Urolithin A supplements to be shockingly below the 100% pure that the labels claim in most cases.
In the past, some manufacturers have been found to sell potent and untested drugs disguised as dietary supplements, posing serious threats to public health. Substances such as Ostarine, DMAA, DMBA, and anabolic steroids in these products posed serious and potentially harmful consequences for consumers[5], [6].
In response to safety concerns, in 2022, the U.S. FDA issued warning letters to various companies for selling adulterated dietary supplements containing unsafe or unauthorised ingredients, some marketed as drugs without proper FDA approval[7].
So, while supplements carry multiple health benefits, it is essential to exercise prudence in our choices and consume high-quality products.
Emphasising the importance of choosing good supplements, it becomes evident that the absence of standardised rules allows any entity to claim their product as “high quality”. Nevertheless, genuine quality in supplements hinges upon the adherence to stringent manufacturing processes, utilisation of pure, potent ingredients, and the absence of harmful contaminants .
Affordability, while noteworthy, should not be the sole determining factor; thus, it is crucial to inquire beyond the label claims when selecting a supplement. Below are main questions to consider:
The Food Standards Agency (FSA) oversees supplement laws in the UK. While there is no mandatory registration or licensing for supplement sale, manufacturers are responsible for adhering to safety and legal standards.
When choosing a supplement, giving preference to products manufactured according to Good Manufacturing Practices (GMP) is essential. GMP encompasses a comprehensive set of regulations that guarantee products' safety, quality, and consistency - from the initial materials to the finished goods.
Manufacturers conduct comprehensive testing on their products as part of GMP compliance and create a Certificate of Analysis (CoA) as evidence of meeting quality standards. This CoA functions as a product report card, providing essential information about its contents and production process. It serves to support the label claim, precisely detailing what the product contains and how it was manufactured.
Following GMP, the next priority usually goes to third-party testing. Unlike in-house testing conducted by the company itself, third-party testing involves an independent organisation evaluating a product or service to ensure it meets specific standards.
As third-party testing is voluntary for supplements choosing third-party tested supplements provides confidence in safety, quality, and adherence to advertised claims.
However, the least scrupulous retailers may just blatantly lie, saying the product is GMP and third-party tested, but keep in mind this quality processing comes at a financial cost, so the very cheapest products are unlikely to use it (whatever they say).
Supplements consist of two types of ingredients: synthetic and natural. Natural nutrients originate from whole food sources, while synthetic nutrients are artificially produced in industrial processes.
The choice between natural and synthetic ingredients in supplements isn't definitively better for one over the other. Each has its advantages based on the context. Synthetic ingredients offer consistency, precise dosing, and fewer contaminants due to controlled manufacturing, making them cost-effective and suitable for some individuals. However, studies on the health benefits of many synthetic nutrients are inconclusive or weak, though not all are harmful.
On the other hand, natural ingredients may contain additional beneficial compounds, have better absorption, and appeal to environmentally conscious consumers. Yet, concerns about microbiological quality exist, stemming from potential bacterial and fungal contaminants in plant-based supplements due to limited industry regulation and quality studies[8]. Additionally, claims of "100% natural" lack a standardised definition.
A common concern revolves around the presence of contaminants. Inadvertently, trace amounts of banned substances like prohormones, hormones, or stimulants can end up in supplements when manufacturing equipment isn't thoroughly cleaned, leading to carryover from previous product batches[9]. This can be avoided through GMP and confirmed through third-party testing.
Bioavailability measures how much of a nutrient from a supplement the body can effectively absorb and utilise. It is influenced not only by natural ingredients but also by synthetic nutrients, which can be tailored for higher absorption rates. Additionally, natural nutrient bioavailability varies with plant source; e.g., carotenoids are more bioavailable from papaya than from tomato and carrot [10].
Several factors influence the bioavailability of supplements:
Formulation: Ingredients, dosage form, and additives affect supplement absorption
Absorption Mechanisms: Supplements are absorbed through various mechanisms, influenced by molecular size, solubility, and the presence of transporters or carrier molecules.
Supplement Format: Different formats, such as tablets, capsules, liquids, or suspensions, can impact how quickly the supplement releases its active ingredients for absorption. Liquid supplements often have faster absorption rates than solid forms due to their rapid dispersion and dissolution in the gastrointestinal tract.
Adjuncts and Excipients: The presence of absorption enhancers, fats and oils, chelated minerals, and co-factors or synergistic nutrients in a supplement can significantly influence the absorption and bioavailability of its active ingredients.
Proper storage and transportation are crucial in maintaining the quality and effectiveness of supplements, as even the best ingredients with high bioavailability can become harmful if mishandled throughout the supply chain.
Here are some key reasons why storage and the supply chain are crucial:
Product Integrity: Temperature, humidity, and light exposure affect supplement stability and potency. Incorrect storage reduces effectiveness over time.
Contamination Risks: A well-managed supply chain minimises exposure to harmful contaminants from manufacturing to warehousing.
Quality Control: Each stage of the supply chain should have quality control measures, including ingredient purity testing, label verification, and quality assurance checks.
Transparency and Traceability: A reliable supply chain ensures compliance with regulations and industry standards, offering transparency and traceability to consumers.
Shelf Life: Proper storage preserves the supplement's shelf life, allowing consumers to use it without compromising quality.
Bright Store Lights: Bright retail store lights can degrade supplement ingredients, cause oxidation in oils, and hinder label legibility.
As exemplified by GMP, continuous safety monitoring and quality control are essential to protect consumers.
Here are several steps you can follow to ensure the supplement you choose is of high quality:
When selecting a supplement, price is not the sole determining factor. In addition to considering affordability, it is crucial to assess the manufacturing process, the ingredients utilised, and the storage methods employed for maximum benefits.
REMEMBER: If the price is too good to be true, the contents probably aren’t.
Blog written by Sanjana Gajbhiye
1. Martínez, Miguel, F Mata, Miguel Angel, Manuel Cansino, Asier Martínez Segura, Antonio Oliver, and Juan M Cortell-Tormo. “Fraud in Nutritional Supplements for Athletes: A Narrative Review,” January 1, 2021. Nutrición Hospitalaria
2. Lauritzen, Fredrik. “Dietary Supplements as a Major Cause of Anti-Doping Rule Violations” 4 (March 25, 2022). Frontiers in Sports and Active Living
3. Pedro Antonio Navarro, Isabel Sospedra, Alejandro Perales, Cristina González-Díaz, Rubén Jiménez-Alfageme, José S Câmara, Angel Gil-Izquierdo, and Miguel Martínez. “Caffeine Health Claims on Sports Supplement Labeling. Analytical Assessment according to EFSA Scientific Opinion and International Evidence and Criteria” 26, no. 7 (April 6, 2021): 2095–95. molecules
5. “Potent and Untested Drugs Sold as ‘Dietary Supplements.’” Core.ac.uk, 2015.
6. “The Era of Fake Medicines: Investigating Counterfeit Medicinal Products for Erectile Dysfunction Disguised as Herbal Supplements” 617 (April 1, 2022). International Journal of Pharmaceutics
7. “Warning Letters to Companies for Selling Adulterated Supplements.” U.S. Food and Drug Administration, 2022.
8. Ratajczak, Magdalena, Dorota Kaminska, Agata Swiatly, and Leszek Pawelczyk. “Quality of Dietary Supplements Containing Plant-Derived Ingredients Reconsidered by Microbiological Approach” 17, no. 18 (September 18, 2020): 6837–37. International Journal of Environmental Research and Public Health
9. “Contamination of Nutrition Supplements.”, October 5, 2020. mysportscience
10. “Carotenoids Are More Bioavailable from Papaya than from Tomato and Carrot in Humans” ResearchGate. Cambridge University Press (CUP), August 12, 2013.
11. “Synthetic vs Natural Nutrients: Does It Matter ?” Healthline - August 17, 2016
Click on resource name for more details.
Ensuring safety of drugs, medical supplies and food which is used daily.
Exponential Increase in Mortality and Diseases
Highlights from Longevity Summit Dublin 2023
Many biomarkers decline with age, but can you supplement to compensate and recover your health?
Including camel's milk, colostrum, hyaluronic acid, and supplements for over 50s and to reduce cholesterol