Clock Foundation’s GrimAge Biological Age Test Review
Epigenetic age test provides information beyond biological age
Join the club for FREE to access the whole archive and other member benefits.
By Michaela Diakatou
Our DNA is organized in the form of chromosomes and the very edge of a chromosome is called a telomere. Telomeres protect chromosomes the way plastic edges protect shoelaces from fraying. Telomeres contain thousands of repeats of a specific DNA sequence (TTAGGG) and are stabilized by a protein complex called “shelterin”.
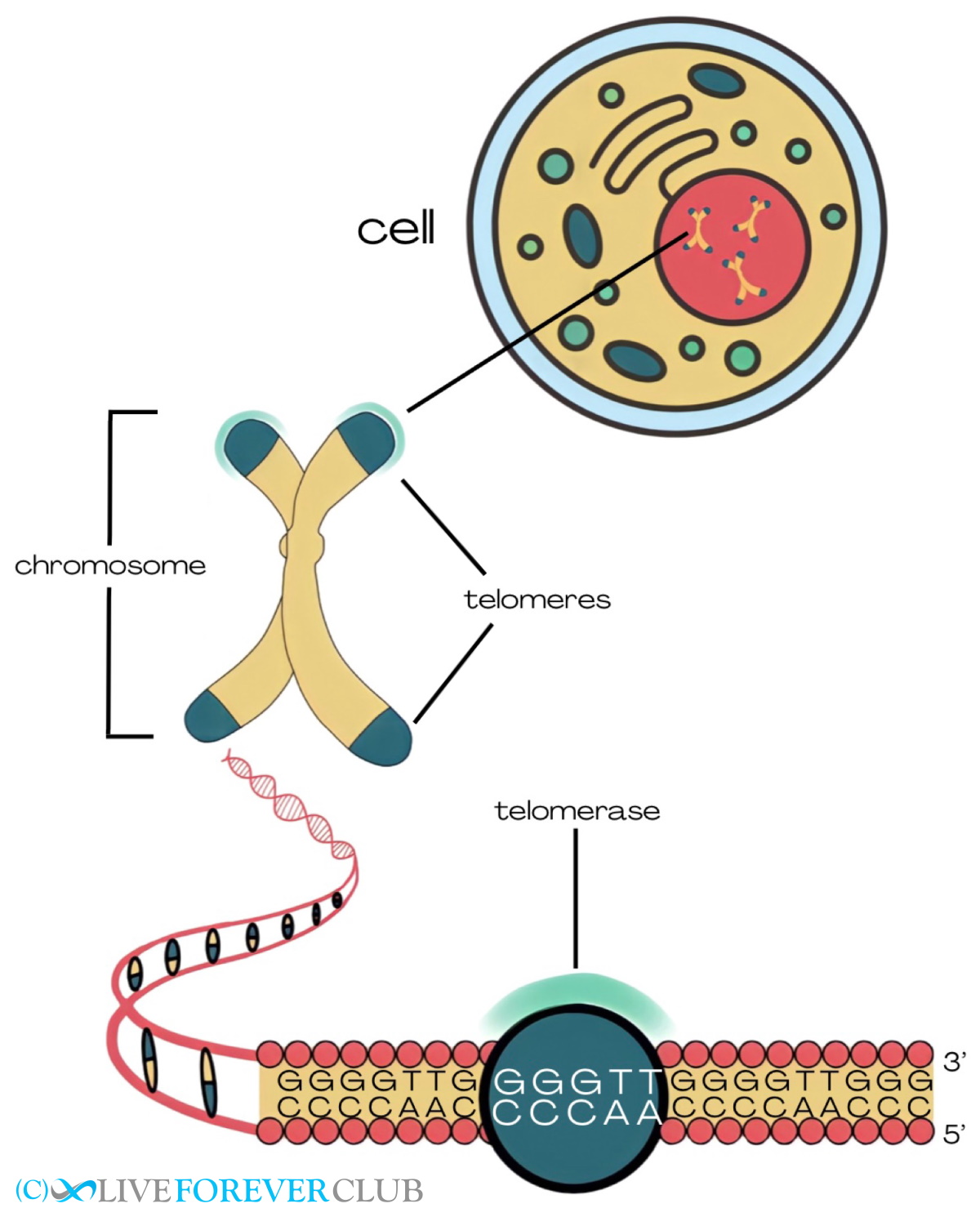
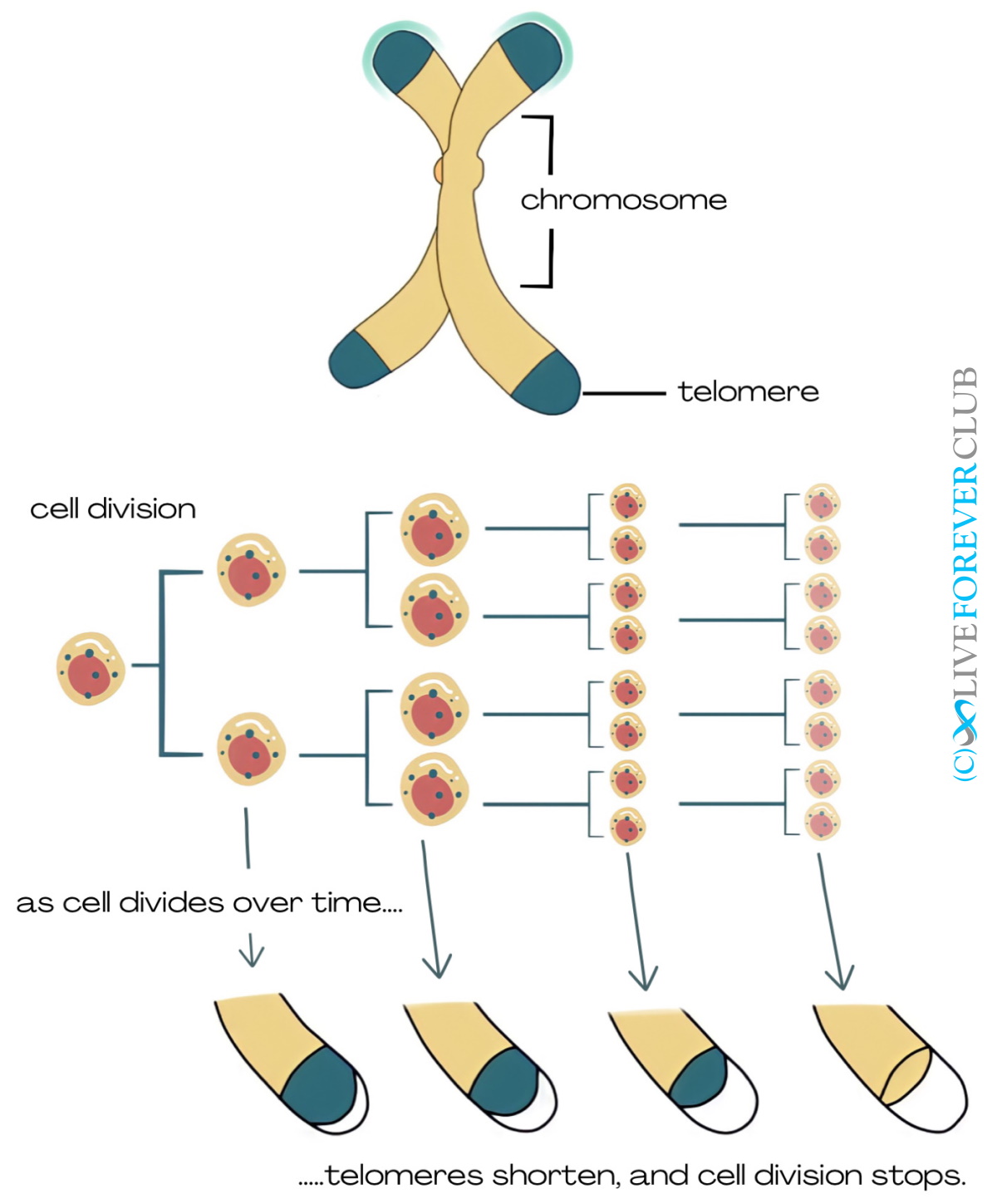
With each cell cycle, the DNA is duplicated and then divided into the daughter cells. However, the cell cannot duplicate the DNA to the very end of each chromosome, so telomeres shorten a little bit with each cell division. The only cells that escape this telomere attrition are germ cells (ovaries and sperm) and stem cells that need to give rise to young cells with full telomeres. The way to evade telomere attrition is by elongating the telomeres after each cell cycle with the enzyme telomerase.
The rest of our cells that do not have that rebuilding “privilege” can only divide a certain number of times before their telomeres become too short. This is known as the Hayflick limit and it is around 50 divisions in human cells[1] Once a cell reaches that limit, it becomes senescent, meaning that it stops dividing and acquires a distinct genetic, epigenetic and metabolic profile that contributes to tissue dysfunction[2].
As described earlier, telomere shortening functions as a molecular “clock” that ticks every time a cell divides. Therefore, many consider telomere length a marker of longevity and mortality, more so than chronological age[3]. In addition, telomere length correlates with health conditions such as cardiovascular diseases. There is data on correlation with other diseases such as neurodegenerative disorders and cancer but they are not consistent[4].
Despite the enthusiasm surrounding telomere length as a marker for longevity, and the bloomed market that accompanies it, the scientific community remains divided. The link between shorter telomeres and increased mortality is not supported by all studies in the field. As a result, it is debatable whether telomere length qualifies as a true biomarker for ageing[4]. According to the American Federation for Aging Research, a valid ageing biomarker needs to (www.afar.org):
1) predict life expectancy better than chronological age
2) monitor general processes behind ageing
3) be measured without causing harm
4) be testable in both laboratory animals and humans
Telomere length satisfies the last two criteria but its accordance with the first two remains questionable[5].
One more argument in this direction is the fact that telomere length is not homogeneous among different tissues of the same person. In fact, in some tissues, telomeres did not shorten at all, as a recent study showed[6,7]. This was expected for the testis which is a reproductive organ but not for the cerebellum, the part of the brain responsible for movement and coordination. The other good news of the study was that despite the wide range of telomere length in different tissues, blood cells that are mostly used in telomere testing, are a good representative of telomere length.
When it comes to telomeres, absolute size is not all that matters. Another important factor is the telomere attrition rate. In fact, when comparing different species, the attrition rate is a better predictor of lifespan than telomere length [8,9]. To illustrate, even though humans have much shorter telomeres than mice (5-15kB compared to 50 kB) they live much longer. Human telomeres may start shorter but their attrition rate is at a much slower pace than mice (around 70bp per year compared to around 7,000bp per year). Therefore, the attrition rates between these two species corresponds better to the lifespan than absolute length. The same conclusion was reached in a hallmark study that compared nine mammalian and bird species with a wide range of lifespan and body mass. The telomere shortening rate was a good predictor of a species’ lifespan, but not the initial telomere length (Whittemore 2019).
Another critical aspect of telomeres is how healthy they are. In other words, how much stress damage they accumulate. Damage-inducing stress can be cell intrinsic (like oxidative damage) or extrinsic (like ionizing radiation). Stress does accelerate the shortening of telomeres and cell senescence. However, long telomeres can also induce senescence if they have accumulated significant damage that remains uncorrected 2. This way, genetically unstable cells are not allowed to replicate, even if their telomeres are still long.
Finally, the shape of telomeres is also crucial. Healthy telomeres form loops (also known as t-loops)[10]. When telomeres are damaged, they become linear and activate the DNA Damage Repair (DDR). This is done through ATM (ataxia-telangiectasia mutant) activation[11]. ATM is a major orchestrator of DSB (Double Strand Break) repair and it is required for the extension of the repeats in the telomeres[12].
Telomere testing has become a popular trend. But how informative is it really? To begin with, telomere testing comes in different methods and prices. Unfortunately, the accuracy remains very low and so does the consistency between different methods and labs.
A common approach to measuring telomere length and the most inexpensive one is qPCR. Tests based on qPCR are those from SpectraCell and TeloYears. A caveat of the qPCR-based methods is that it measures the average length of all telomeres and cannot detect the shortest telomeres[13]. However, a chain is only as strong as its weakest link and it is the shortest telomeres that will define how soon senescence will be induced. Another downside of qPCR analysis is that the results are dependent on small alterations of experimental parameters (like salt concentration or temperature) which are challenging to keep identical for every blood sample. As a result, the output can be very inconsistent, especially between different labs.
A more precise method than qPCR, but still not fully accurate, is FISH (fluorescent in situ hybridization) also seen as qFISH (quantitative FISH) and Flow-FISH . FISH is used for the tests Life Length and RepeatDx. In contrast to qPCR, FISH detects the length of each telomere separately; so the shortest telomeres are also analyzed[13].
Finally, the method considered the gold standard for telomere length measurement is Terminal Restriction Fragment (TRF). With TRF all telomeres run separately on an electrophoresis gel, so each one is visualized separately. The downside of TRF is that it is only qualitative, despite efforts to quantify the results[14].
There is a plethora of ageing biomarkers other than telomere length. The most common ones are DNA methylation, transcriptomic, proteomic and metabolomic predictors. The combination of different biomarkers into one predictor is called a composite biomarker predictor. Arguably, the most predictive biomarker for biological age is DNA methylation age (DNAm age), also known as an epigenetic clock[4,15]. Methylation is the addition of -CH3 groups to the DNA and it is used to control gene expression by turning genes “on and off”. Methylation patterns change with age and this is what DNAm age measures.
The big question is how do all these ageing biomarkers compare to each other? This is what a study that measured eleven markers of biological age in 1037 people tried to answer[16]. The markers were telomere length and attrition, three epigenetic clocks and their ticking rates and three composite biomarkers (KDM biological age, age-related homeostatic dysregulation, and the pace of ageing). The authors examined the relationship between the different markers and ageing-related outcomes (physical condition, cognitive function and subjective perception of ageing).
The results showed that the markers with the best association, albeit light, with ageing outcomes, were one epigenetic clock (the 71–cytosine-phosphate-guanine clock) and the composite biomarkers. Telomere length and attrition were only weakly associated with the pace of ageing and not associated with the other signs of ageing. This agrees with more studies in the field. Furthermore, the different types of markers were not in agreement with each other. That probably shows that they measure different aspects of the ageing process.
It is inevitable that the length of telomeres is related to age and some health aspects. However, how representative it is of mortality and overall health is debatable. Telomere length varies in different tissues and other aspects of telomeres like attrition rate and accumulated damage are also important. On top of that, the current methods for testing telomere length are not accurate enough, and results vary between different methods and labs.
That does not mean that testing has no value. What one could do is get tested at different points of time, for example every few years, and observe how their results progress in time. Ideally, for comparison purposes, tests from different labs would be used at the same time (rumour has it, it is better to use different names!).
Let’s keep in mind though, that epigenetic clocks are better predictors of ageing signs, at least for now. So, epigenetic clock testing would be the first choice and if one could afford it, they could accompany it with telomere testing, for a more complete picture of the ageing process.
1. The Hayflick Limit | The Embryo Project Encyclopedia. Available at: https://embryo.asu.edu/pages/hayflick-limit. (Accessed: 14th August 2022)
2. Victorelli, S. & Passos, J. F. Telomeres and Cell Senescence - Size Matters Not. EBioMedicine 21, 14–20 (2017).
3. Shekhidem, H. A. et al. Telomeres and Longevity: A Cause or an Effect? Int. J. Mol. Sci. 20, (2019).
4. Vaiserman, A. & Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Genet. 11, (2021).
5. Sanders, J. L. & Newman, A. B. Telomere length in epidemiology: a biomarker of aging, age-related disease, both, or neither? Epidemiol. Rev. 35, 112–131 (2013).
6. Demanelis, K. et al. Determinants of telomere length across human tissues. Science (80-. ). 369, (2020).
7. Telomere length varies across human tissue types -- ScienceDaily. Available at: https://www.sciencedaily.com/releases/2020/09/200910150320.htm. (Accessed: 14th August 2022)
8. Tricola, G. M. et al. The rate of telomere loss is related to maximum lifespan in birds. Philos. Trans. R. Soc. B Biol. Sci. 373, (2018).
9. Whittemore, K., Vera, E., Martínez-Nevado, E., Sanpera, C. & Blasco, M. A. Telomere shortening rate predicts species life span. Proc. Natl. Acad. Sci. U. S. A. 116, 15122–15127 (2019).
10. Breakthrough could impact cancer, ageing and heart disease. Available at: https://medicalxpress.com/news/2018-07-breakthrough-impact-cancer-ageing-heart.html. (Accessed: 14th August 2022)
11. Van Ly, D. et al. Telomere Loop Dynamics in Chromosome End Protection. Mol. Cell 71, 510-525.e6 (2018).
12. Lee, S. S., Bohrson, C., Pike, A. M., Wheelan, S. J. & Greider, C. W. ATM kinase is required for telomere elongation in mouse and human cells. Cell Rep. 13, 1623 (2015).
13. Lulkiewicz, M., Bajsert, J., Kopczynski, P., Barczak, W. & Rubis, B. Telomere length: how the length makes a difference. Mol. Biol. Rep. 47, 7181–7188 (2020).
14. Greenfield Ben, A. B. Ben Greenfield Life Podcast. September (2019).
15. Jylhävä, J., Pedersen, N. L. & Hägg, S. Biological Age Predictors. EBioMedicine 21, 29–36 (2017).
16. Belsky, D. W. et al. Eleven Telomere, Epigenetic Clock, and Biomarker-Composite Quantifications of Biological Aging: Do They Measure the Same Thing? Am. J. Epidemiol. 187, 1220–1230 (2018).
Click on resource name for more details.
Charitable organization with mission to provide funding for biomedical research on aging
Laboratories offering tests for nutritional deficiencies, cardiovascular risk and genetic testing
Arise Sir-Tuin: Why sirtuins are the knights battling ageing
Effects of Whole Body Cryotherapy (WBC)
Epigenetic age test provides information beyond biological age
What can your glycome tell you about your true age?
Adrian tries out this home epigenetic test and is impressed with the level of detail in the reports
How does the cheapest epigenetic age test on the market compare?