Measuring the hallmarks of ageing – how can you track the underlying causes of ageing?
What consumer tests are available to check your hallmarks of ageing and how they're impacting your body?
Join the club for FREE to access the whole archive and other member benefits.
Sirtuins are proteins with a variety of functions, all of which are linked to one very interesting outcome – they influence life spans.
When the first sirtuin was discovered in yeast in 1979, it was rather boringly labeled ‘mating type regulator-1’ or MAR-1. Yeast cells that didn’t have this protein were sterile as their key mating genes were silenced. Later that same year, when more proteins with similar functions were discovered, they were clubbed into a family that was relabeled as ‘silent mating type information regulation 2 homolog’ or ‘sirtuin’.
It was only twenty years after their first discovery, that sirtuins were discovered to be molecular fountains of youth. This was found in a seminal study in 1999 which showed that overexpressing the sir2 gene in yeasts could extend the organism’s lifespan by up to 70%.
Now, we know that the anti-ageing function of sirtuins is widespread in most organisms – ranging from single-celled yeasts to multicellular creatures like flies, round worms, and even mammals.
Functionally, sirtuins are enzymes catalyzing chemical reactions in the cell.
In yeast, sir2 exercises its anti-ageing function by increasing the cell’s genomic stability. Sir2, along with several other proteins, is involved in deacetylating histones, which are proteins around which DNA is wrapped to form compact structures within the nucleus. Wherever such deacetylation of histones occurs, the DNA in that area becomes tightly packed and thereby ‘silenced’. Sir2 plays a crucial role in ‘silencing’ parts of the yeast genome that are prone to unwanted recombination.
During normal recombination, DNA molecules break up and rejoin with other DNA pieces in a controlled manner for repair or to form new combinations of genetic material during sexual reproduction. However, in yeast, certain regions of the genome undergo uncontrolled recombination; this pinches off pieces of the yeast’s chromosomes into toxic extrachromosomal DNA circles that are a leading cause of yeast ageing.
Any reductions in sir2 levels or mutations to the sir2 gene that lead to a loss of its function, cause the numbers of these toxic extrachromosomal DNA circles to skyrocket, leading to early ageing and shortened life spans.
In addition to its genome-stabilizing activity, sir2 is also essential for effecting the increased lifespan associated with calorie restriction. Experiments have shown that during calorie restriction, levels of sir2 rise, which likely contributes to the extension of life span; however, the exact mechanisms involved in this process are not yet fully understood.
Unlike yeast sirtuins, the mechanisms by which mammalian sirtuins work to keep ageing at bay are different and much more complicated.
Mammals are known to have 7 sirtuin genes labeled SIRT1–SIRT7. Of these, SIRT1 and SIRT2 are found in both the nucleus and cytoplasm; SIRT3, SIRT4, and SIRT5 are found in the mitochondria; and SIRT6 and SIRT7 are found only in the nucleus.
Of all the 7 SIRTs in mammals, SIRT1 is the best studied, and this sirtuin is known to be involved in a multitude of processes. Most mouse embryos that lack the SIRT1 gene (nearly 80%) do not survive; the 20% that do survive suffer from developmental abnormalities and are usually sterile and much smaller than normal mice.
Mice with extra copies of the SIRT1 gene live longer than normal mice and suffer from lower levels of DNA damage as they age. Decreases in SIRT1 levels are almost always correlated with ageing in a variety of tissues and cells. Overexpression of SIRT1 in heart cells (cardiomyocytes) facilitates their recovery from heart attacks, and activation of the gene delays ageing in a variety of cells such as fibroblasts and smooth muscle cells. SIRT1 is also known to affect cellular metabolism, resistance to stress, and can modulate the immune system. It is also involved in stalling cell death mechanisms to facilitate DNA repair and helps maintain genome integrity especially under stress. SIRT1 expression has been linked to the survival of pancreatic β-cells (which secrete insulin) under stress and is also known to be involved in the survival of neurons through its protective effects against neurodegeneration in mouse models of Alzheimer’s and Parkinson’s disease.
SIRT2, which is mainly found in the cytoplasm of the cell, is known to regulate the cell cycle and works with SIRT1 to ensure that chromosomes are tightly condensed during cellular reproduction.
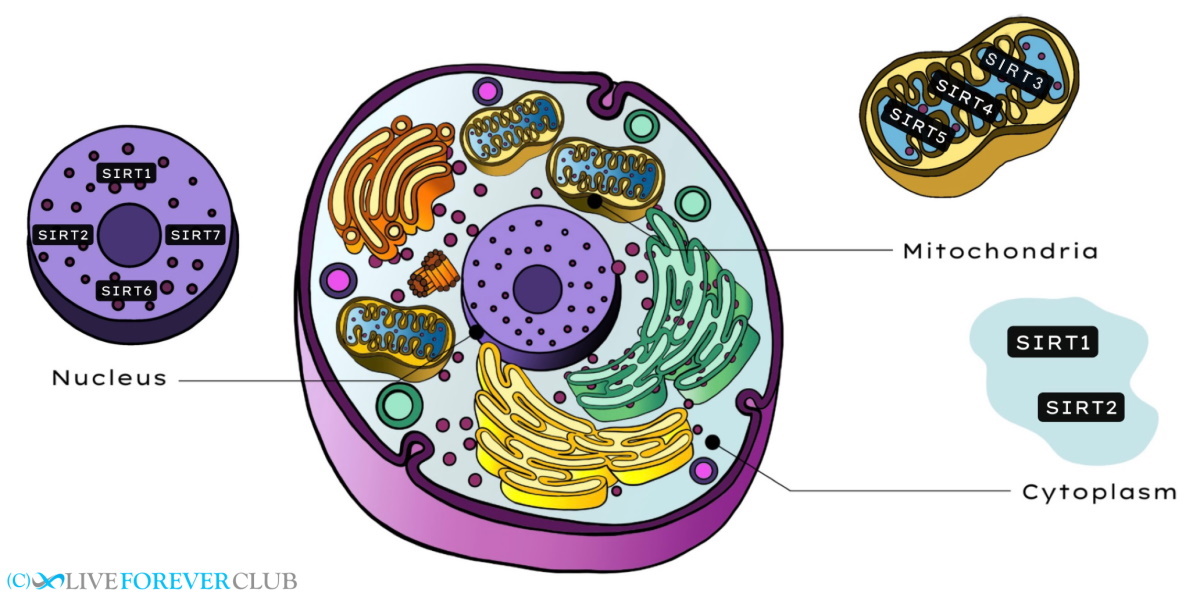
Of the three sirtuins (SIRT3, SIRT4, and SIRT5) found in the mitochondria (energy producers or powerhouses of the cell), SIRT3 is the best studied, and is the only known Sirtuin to affect human longevity. However, although there is some genetic evidence which suggests that certain variants (or polymorphisms) of the SIRT3 gene are associated with long life, this has yet to be proved conclusively. Mice lacking the SIRT3 gene consume less oxygen but produce more reactive oxygen species (ROS) than normal mice; SIRT3 deficient mice, therefore, suffer more DNA damage during ageing than normal mice. SIRT4 plays an important role in modulating energy use in the body as it is involved in regulating insulin secretion and may mediate metabolic changes associated with calorie restriction. SIRT5 is the least characterized of all the mammalian Sirtuins and its function is as yet unknown.
SIRT6 and SIRT7, which are found exclusively in the nucleus, have been identified as being indispensable for normal life spans. Mice depleted of SIRT6 begin to show signs of degeneration and premature ageing just 3 weeks after birth and usually die by 4 weeks of age. These effects seem to be caused by severe problems in glucose metabolism at an organismal level and increased genomic instability (chromosome fragmentation, loss, etc.) at the cellular level, especially in dividing cells. Mice lacking the SIRT7 gene also show progeria (early onset of ageing) and eventually die of cardiac issues.
SIRT1, SIRT2, and SIRT6 are involved in DNA packaging (through a process called chromatin condensation) by deacetylating histones. SIRT1 is also known to be involved in regulating histone gene expression, and during oxidative stress, reduces the expression of ribosome-forming genes and protein production in general. This helps in protecting the cell from energy deprivation and facilitates repair.
SIRT1 is also found to be localized in pericentromeres (regions important for chromosome movement during cell division) and in telomeres (end regions of chromosomes whose shortening is closely correlated to ageing). SIRT1 overexpression in mice inhibits telomere erosion and silencing causes enhanced telomere erosion. Another sirtuin that binds and stabilizes the telomeric regions is SIRT6, especially during cell reproduction or during stressful conditions involving DNA damage. In addition to their effects on DNA packaging and gene expression, SIRT1 and SIRT6 also interacts with many proteins to inhibit apoptosis (cell death) and promote DNA repair.
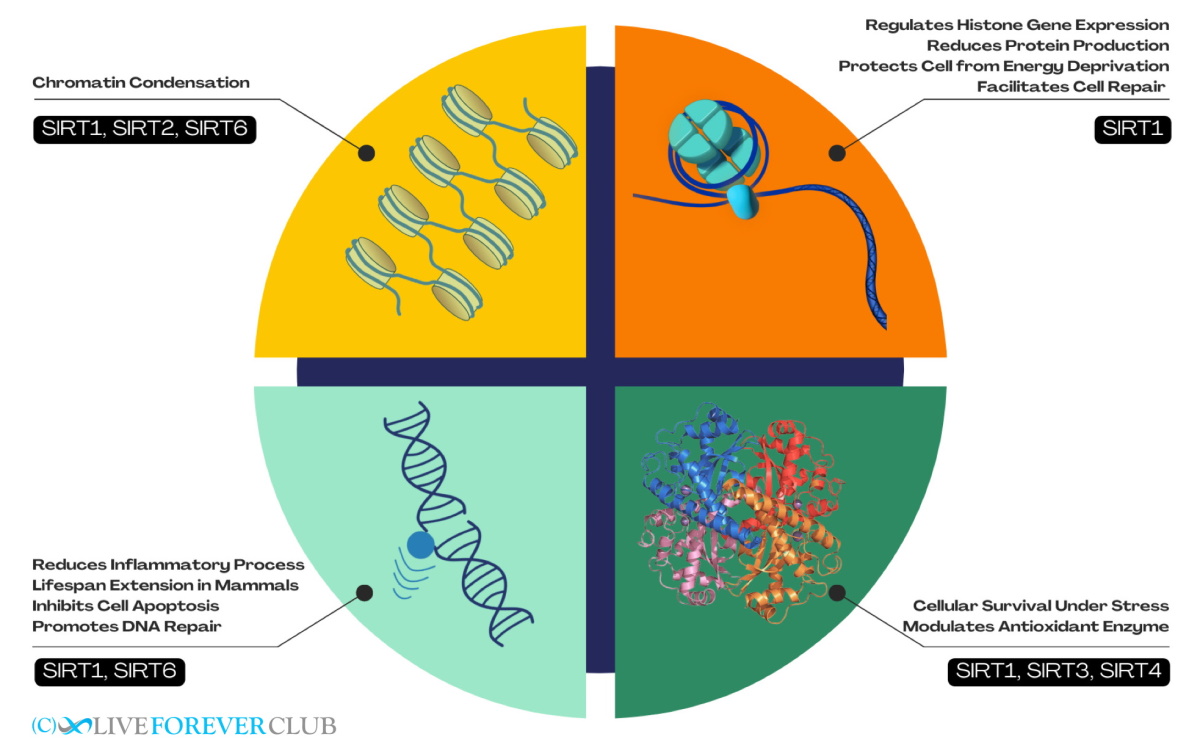
Apart from their function in maintaining genome integrity, sirtuins are also intimately involved with metabolism. SIRT3 and SIRT4, along with SIRT1 are essential for cellular survival under oxidative and metabolic stresses. SIRT1 and SIRT3 have been shown to be involved in counteracting oxidative stress by modulating the activity of antioxidant enzymes. SIRT1 and SIRT2 interact with several members of the FOXO (forkhead box O) protein family, which are a group of gene regulators known to promote longevity in mammals through an as-yet unknown mechanism.
Finally, it has also been found that SIRT1 and SIRT6 influence the immune system via their interactions with NFκ-B (Nuclear Factor Kappa-B), a well-known mediator of inflammation. Through these interactions, the sirtuins reduce inflammatory processes and contributes to lifespan extension in mammals.
As of now, there are two known methods by which sirtuins can be activated to promote longevity, namely, dietary restriction (or calorie restriction) and STACs (Sirtuin-Activating Compounds).
Dietary or calorie restriction (by 20–50%) has been shown to enhance life span in many organisms, including yeast, fruit flies, fish, rats, hamsters, mice, and dogs by as much as 50%. In many of these organisms, the beneficial effects of calorie restriction seem to be mediated by sirtuins, especially SIRT1 and SIRT6, both of which seem to work in tandem to reduce inflammation-related ageing processes. Furthermore, sirtuins also seem to have beneficial effects on stalling age-related changes to the cardiovascular system via their effects on glucose and fat metabolism. However, since long-term calorie restriction can have many undesirable side effects, researchers are currently focused on discovering and understanding how sirtuins can be activated pharmaceutically with the help of a variety of natural compounds.
As of now, a plethora of plant-derived substances, such as flavones, anthocyanidins, polyphenols (such as curcumin and resveratrol), to name a few, are being tested as sirtuin activators. Currently, resveratrol (a compound found in the skin of grapes and red wine) is one of the most promising candidates as a lifespan improving agent due to its potent SIRT1-activation effects. Resveratrol has been shown to improve both, the lifespan and health of several species, including mice and rats, by mimicking the molecular effects of calorie restriction by activating SIRT1. This translates to resveratrol showing strong neuroprotection, antitumor, and antioxidant activities.
Besides this, the lifespan-enhancing effects of many plant-based natural compounds, melatonin, and synthetic sirtuin activators via their effects on the other sirtuins such as SIRT3 and SIRT6 are also being studied. Although the therapeutic potential of these compounds in humans is yet to be clinically validated, studies on cell cultures and in animals such as mice and rats have been showing interesting anti-ageing results.
Currently, gene therapy approaches to boost sirtuin activity in heart cells, liver cells, and even whole organisms such as mice are showing very promising results. Perhaps not too far in the future, a combination of genetic and dietary therapies can not only make us live longer, but also maintain good health and reduce frailty during later years of life.
Blog written by Anusha Krishnan
Grabowska, W., Sikora, E. and Bielak-Zmijewska, A., 2017. Sirtuins, a promising target in slowing down the ageing process. Biogerontology, 18(4), pp.447-476.
Guarente, L., 2007, January. Sirtuins in aging and disease. In Cold Spring Harbor symposia on quantitative biology (Vol. 72, pp. 483-488). Cold Spring Harbor Laboratory Press.
What are Sirtuins and what do they do? https://www.lifespan.io/topic/what-are-sirtuins-and-what-do-they-do/ (accessed 2nd Aug 2022)
Mohar, D.S. and Malik, S., 2012. The sirtuin system: the holy grail of resveratrol?. Journal of clinical & experimental cardiology, 3(11).
Merksamer, P.I., Liu, Y., He, W., Hirschey, M.D., Chen, D. and Verdin, E., 2013. The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY), 5(3), p.144.
Exhibitor highlights from Health Optimisation Summit 2022
Do telomere length tests really reveal your biological age?
What consumer tests are available to check your hallmarks of ageing and how they're impacting your body?
The biology of ageing looks at way more than just the genome and microbiome - here's 20 to start with!
How the observation and understanding of smaller and smaller biological units has improved ageing research and medicine
Absolute numbers look good, but for the size and proximity of the venue, it could have done better
Key points and slides from the leading longevity conference hosted by Aubrey de Grey