Meet the Omes
The biology of ageing looks at way more than just the genome and microbiome - here's 20 to start with!
Join the club for FREE to access the whole archive and other member benefits.
In business, it's often said, "If you can't measure it, you can't manage it." This means tracking progress is essential for success. The same concept applies to our health and ageing. To manage the effects of ageing, we must first find ways to measure key signs. Currently, there are 12 known hallmarks of ageing.
The table below summarizes which hallmarks of ageing can be measured with consumer tests.
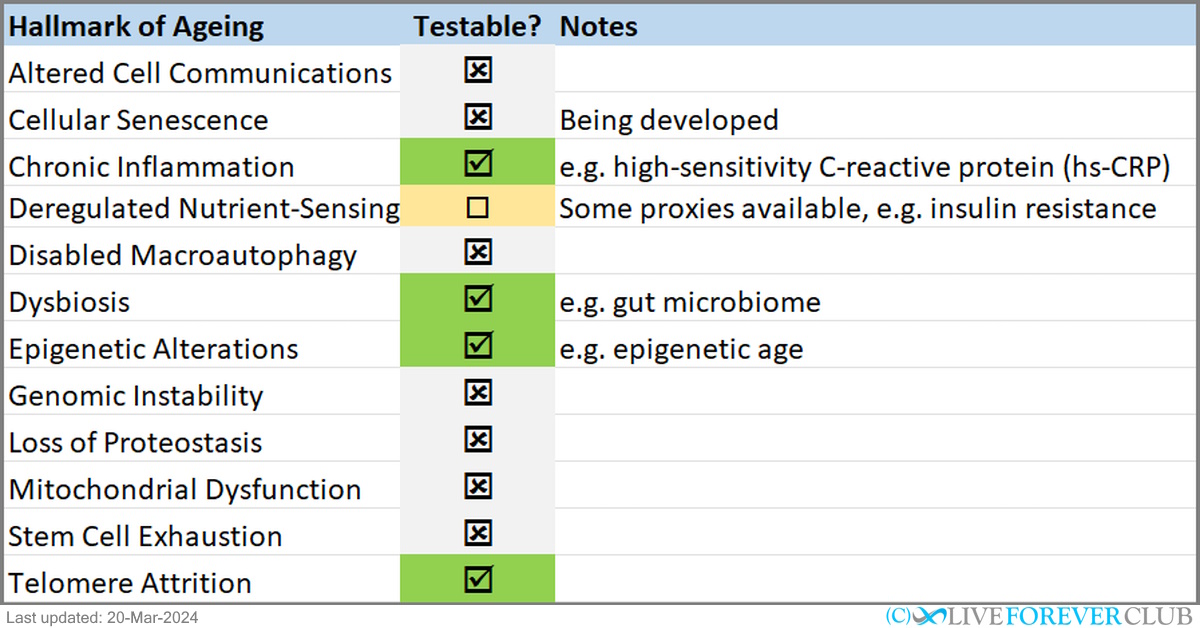
Let's explore what they are and how can we measure them.
As we age, our cells experience interruptions in communicating. This is called altered cell communication, a major reason we experience health problems as we age.
Understanding exactly how this communication shifts is difficult. Complex pathways and constantly changing interactions between cells make it a big challenge. Every day, cells send millions of messages through different methods, such as chemical messengers in the blood or direct contact. To study this process, scientists often need to separate cells, add specific chemicals to change their behaviour, and then use powerful tools like microscopes and gene analysis machines to see how they respond. This approach is too complex for a simple, routine test. Hence, there are currently no tests that explicitly measure the rate of cell communication.
Our cells have a built-in limit on how many times they can divide. When they reach this limit, they enter a state called "cellular senescence" where they stop dividing but stay alive and release active molecules. Our immune system is constantly working to identify and clear these senescent cells. This clearance process isn't always perfect, hence, some senescent cells may accumulate over time.
Understanding how our body removes old cells or measures their accumulation is important. However, today, there are no direct tests available to measure senescent cells. This is because of its complex nature and many different biomarkers associated. Biopsies are the most reliable method but are invasive and impractical for routine testing. Scientists are increasingly adopting specialised techniques, for example, staining, flow cytometry and gene analysis, to identify the senescent cells.
Chronic inflammation is increasingly recognized as a critical hallmark of ageing. Unlike acute inflammation, which is a beneficial and temporary response to injury or infection, chronic inflammation is a low-grade, persistent immune response that damages tissues over time. Chronic inflammation is linked to conditions such as cardiovascular diseases, diabetes, cancer, arthritis, and neurodegenerative disorders.
To understand the extent and impact of chronic inflammation in the body, measuring inflammation levels is essential. Doctors use various techniques, including detecting inflammatory biomarkers in blood tests. Commonly measured markers include C-reactive protein (CRP), interleukin-6 (IL-6), tumour necrosis factor-alpha (TNF-α), and high-sensitivity C-reactive protein (hs-CRP) – used for more sensitive detection of low-grade inflammation. These measurements provide insight into an individual's inflammatory status and help predict the risk of developing age-related diseases.
Consumer tests for measuring inflammation levels have become more accessible. These allow individuals to monitor their inflammation status from home. Typically, they measure biomarkers like CRP through blood samples collected via finger pricks. For optimal health, aim for CRP <1.0 mg/L, hs-CRP <2.0 mg/L, IL-6 <5 pg/mL, and TNF-α <8 pg/mL.
Cells and organisms have a built-in system for understanding their nutrient intake and energy levels. This system, called nutrient-sensing, plays a vital role in keeping the body functioning well. In younger individuals, this system precisely adjusts how the body uses energy, grows, and repairs cells based on what's eaten.
As we age, however, this system can malfunction. This malfunction is called deregulated nutrient-sensing, and it disrupts the body's ability to respond effectively to food and energy. This can lead to a number of problems including difficulty managing blood sugar levels (diabetes), excessive weight gain (obesity), and an increased risk of developing cancer.
Currently, there are limited options for consumers to directly assess their nutrient-sensing function. Continuous glucose monitors (CGMs) and home insulin resistance tests could offer a glimpse into how well the body responds to dietary intake, but they capture only a part of the broader nutrient-sensing picture.
Our cells have a cleaning system called macroautophagy, often known as autophagy. This process continuously removes damaged parts and misfolded proteins, like taking out the trash. It's crucial for keeping cells healthy, especially under stress, by getting rid of harmful junk. As we age, this cleaning system becomes sluggish, leading to a buildup of cellular waste and potentially causing age-related diseases like Alzheimer's, cancer, and diabetes.
Monitoring macroautophagy is difficult for consumers due to several scientific, technical, and practical limitations. The techniques used to assess macroautophagy, like western blotting and electron microscopy, are intricate and expensive. These methods are not suitable for simple at-home testing kits. Importantly, there are no easy-to-measure indicators in the body fluids that directly reflect macroautophagy activity in tissues. Existing markers require analysing cells directly, which is impossible with current consumer health test technologies.
Dysbiosis is an imbalance in the communities of microbes that live in our bodies, especially in the gut. This gut community contains both helpful and harmful microbes. As we age, our lifestyle, diet, antibiotics, and other bodily changes can throw this balance off. This imbalance, or dysbiosis, can lead to a variety of health problems common in older people. These problems include digestive issues, a weaker immune system, constant inflammation, and a higher chance of infection.
There are ways to measure dysbiosis by looking at what the gut bacteria are made of and how they function. Scientists use techniques such as 16S ribosomal RNA sequencing and metagenomic sequencing to examine the population.
Consumers can now find tests that can provide information about their own gut bacteria. These tests usually require a stool sample and look at the DNA of the gut microbes. This gives a picture of what kinds of bacteria are there and how diverse the gut microbiome is. Testing kits are available from many companies including Chuckling Goat and Randox Health. Generally, the more diverse the gut microbiome, the better the health.
DNA holds all the instructions for how the body functions. However, certain chemical changes like DNA methylation, histone modifications, and other alterations resulting from unhealthy lifestyles can change how the body interprets these instructions. This leads to modifications in gene expression known as epigenetic alterations.
Epigenetic testing kits are becoming more prevalent, offering individuals a glimpse into their biological age and potential health risks. Some of the at-home kits are provided by Clock Foundation, myDNAge and TruDiagnostic (TruAge). These tests involve collecting samples at home (often saliva or cheek swabs) and sending them for analysis.
The tests focus on specific DNA regions with epigenetic changes, like DNA methylation. Diverse techniques like bisulfite sequencing, pyrosequencing, and mass spectrometry are used to analyse the patterns. Bisulfite treatment is essential in many techniques because it helps differentiate between different types of DNA regions. This process allows for the precise assessment of the altered patterns and enhances our understanding of epigenetic regulation throughout the genome. Ideally, in a healthy individual, the biological age determined by epigenetic tests would be less than their chronological age.
Genomic instability refers to an increased rate of mutations within a cell's DNA. These mutations can be single-letter typos or large-scale rearrangements of chromosomes. This instability is particularly relevant to ageing as it disrupts normal cellular functions and can lead to age-related diseases.
Currently, consumer tests for genomic instability are limited. Some companies offer tests for specific signs of DNA damage, but they don't give the whole picture. This is because genomic instability arises from a complex interplay of factors, making a single test insufficient. Clinically, measuring genomic instability can be used to assess a person's cancer risk. Since many cancers exhibit high levels of instability, identifying individuals with this predisposition allows for earlier detection and intervention strategies.
Proteostasis, the body's system for managing proteins, weakens as we age. This system normally builds, folds, moves, and breaks down proteins within cells. When it falters, misfolded proteins accumulate. These malfunctioning proteins can damage cells and contribute to diseases such as Alzheimer's, Parkinson's, and Huntington's.
Consumer-friendly proteostasis testing is currently unavailable. This is because it involves many parts working together to maintain protein health. Studying these interactions requires sophisticated biochemical and molecular biology techniques that are difficult to simplify for consumers. Also, unlike common tests like blood sugar or cholesterol, a proteostasis test wouldn't directly tell you how to improve your health. Without a clear connection between the test results and how to improve your health, most people likely wouldn't be interested in getting tested.
Our cells have tiny structures called mitochondria that act like power plants, turning nutrients into energy. However, these power plants can weaken over time, producing less energy and harmful byproducts. This decline in function is called mitochondrial dysfunction. Mitochondrial dysfunction is linked to ageing and various diseases like neurodegeneration, heart disease, and diabetes.
Currently, there are no simple tests for consumers to directly assess their mitochondrial health. However, some companies offer tests that focus on broader health markers indirectly linked to mitochondrial function, such as cell oxidative stress markers and biomarkers of inflammation. For instance, malondialdehyde (MDA) is a by-product of lipid peroxidation and is often used as a marker for oxidative stress. Levels of MDA can be measured in blood or tissue samples.
Stem cells can replenish themselves and transform into many different cell types, making them essential for fixing and rebuilding tissues. But stem cell exhaustion occurs over time; where stem cells slowly become less effective. Meaning, as we age, there are fewer stem cells, and the rest don't work so well either. This impairs tissue repair and makes us more likely to get age-related illnesses.
The exciting field of stem cell research offers hope for combating ageing and disease. Scientists are making significant strides in understanding the causes of stem cell exhaustion and how it contributes to the ageing process. Though consumer tests aren't currently a focus, this research could lay the foundation for future diagnostic tools and treatments.
Every chromosome has special "caps" called telomeres that protect its important components. These caps are vital in the cell division process. However, each time a cell divides, a small portion of the telomere is not fully replicated, leading to gradual shortening. This process, known as telomere attrition, is linked to ageing as shorter telomeres are associated with decreased cell proliferation and potential malfunction.
Before we're born, telomeres are quite long, about 15 kilobases, but they naturally get shorter as cells divide, dropping to around 10 kilobases by birth. This shortening process keeps happening as our cells keep busy with tasks like growing and repairing throughout our lives. As we grow older (around 60-70 years old), telomeres can shorten to about 5 kilobases on average.
Telomere length can be tracked at home with simple finger prick or cheek swab tests. These tests monitor telomere shortening over time, providing insights into your biological age. The samples are then tested in the lab using different techniques. The most popular technique is the Fluorescence in situ hybridization (FISH) in which the experts paint the telomeres with a special dye that lights up under a microscope. This lets them easily see how long the caps are to understand the length of telomeres. Direct to consumer tests are hard to find, but some companies provide telomere length tests through clinics and healthcare professionals.
Studying how we age, from changes in how our cells talk to each other to the shortening of telomeres, shows how complex and connected the biological processes of ageing are.
Right now, there aren't many direct-to-consumer tests for specific causes of ageing, but the world of health testing is changing fast. New developments in biotechnology and a better understanding of the science behind ageing are leading to the creation of at-home tests that offer clues about different parts of the ageing process.
Future tests will likely expand to cover more ageing biomarkers, allowing people to better monitor their health, understand ageing, and make changes to support healthy longevity.
Blog written by Sanjana Gajbhiye.
1. Lifespan Extension Advocacy Foundation. (2021, April 20). Why we Age: Altered Intercellular Communication. Lifespan.io; Lifespan Extension Advocacy Foundation. https://www.lifespan.io/topic/altered-intercellular-communication/
2. Huang, W., Hickson, L. J., Eirin, A., Kirkland, J. L., & Lerman, L. O. (2022). Cellular senescence: the good, the bad and the unknown. Nature Reviews Nephrology, 18(10), 611–627. https://doi.org/10.1038/s41581-022-00601-z
3. Baechle, J. J., Chen, N., Makhijani, P., Winer, S., Furman, D., & Winer, D. A. (2023). Chronic inflammation and the hallmarks of aging. Molecular Metabolism, 74, 101755–101755. https://doi.org/10.1016/j.molmet.2023.101755
4. Lifespan Extension Advocacy Foundation. (2021, April 20). Why we Age: Deregulated Nutrient Sensing. Lifespan.io; Lifespan Extension Advocacy Foundation. https://www.lifespan.io/topic/deregulated-nutrient-sensing/
5. Nieto-Torres, J. L., & Hansen, M. (2021). Macroautophagy and aging: The impact of cellular recycling on health and longevity. Molecular Aspects of Medicine, 82, 101020–101020. https://doi.org/10.1016/j.mam.2021.101020
6. Carter, C. S. (2021). A “Gut Feeling” to Create a 10th Hallmark of Aging. The Journals of Gerontology: Series A, 76(11), 1891–1894. https://doi.org/10.1093/gerona/glab191
7. Wang, K., Liu, H., Hu, Q., Wang, L., Liu, J., Zheng, Z., Zhang, W., Ren, J., Zhu, F., & Liu, G.-H. (2022). Epigenetic regulation of aging: implications for interventions of aging and diseases. Signal Transduction and Targeted Therapy, 7(1). https://doi.org/10.1038/s41392-022-01211-8
8. Lucía López-Gil, Pascual-Ahuir, A., & Proft, M. (2023). Genomic Instability and Epigenetic Changes during Aging. International Journal of Molecular Sciences, 24(18), 14279–14279. https://doi.org/10.3390/ijms241814279
9. Tang, J.-X., & Xiao, F.-H. (2023). Editorial: The regulation of proteostasis in aging. Frontiers in Cell and Developmental Biology, 11. https://doi.org/10.3389/fcell.2023.1221510
10. Miwa, S., Kashyap, S., Chini, E., & Thomas von Zglinicki. (2022). Mitochondrial dysfunction in cell senescence and aging. Journal of Clinical Investigation, 132(13). https://doi.org/10.1172/jci158447
11. Lifespan Extension Advocacy Foundation. (2020, September 10). Why we Age: Stem Cell Exhaustion. Lifespan.io; Lifespan Extension Advocacy Foundation. https://www.lifespan.io/topic/stem-cell-exhaustion/
12. Butler, M. G., Tilburt, J., DeVries, A., Bethi Muralidhar, Aue, G., Hedges, L., Atkinson, J., & Schwartz, H. (1998). Comparison of Chromosome Telomere Integrity in Multiple Tissues from Subjects at Different Ages. Cancer Genetics and Cytogenetics, 105(2), 138–144. https://doi.org/10.1016/s0165-4608(98)00029-6
Supplementing to Survive
Monitoring my blood glucose response with a Freestyle Libre 3 CGM
The biology of ageing looks at way more than just the genome and microbiome - here's 20 to start with!
How the observation and understanding of smaller and smaller biological units has improved ageing research and medicine
Absolute numbers look good, but for the size and proximity of the venue, it could have done better
Key points and slides from the leading longevity conference hosted by Aubrey de Grey