A paper published in the journal Aging looks at recent developments in our understanding of epigenetics, and whether the DNAm clock is only one component of a more modular ageing mechanism.
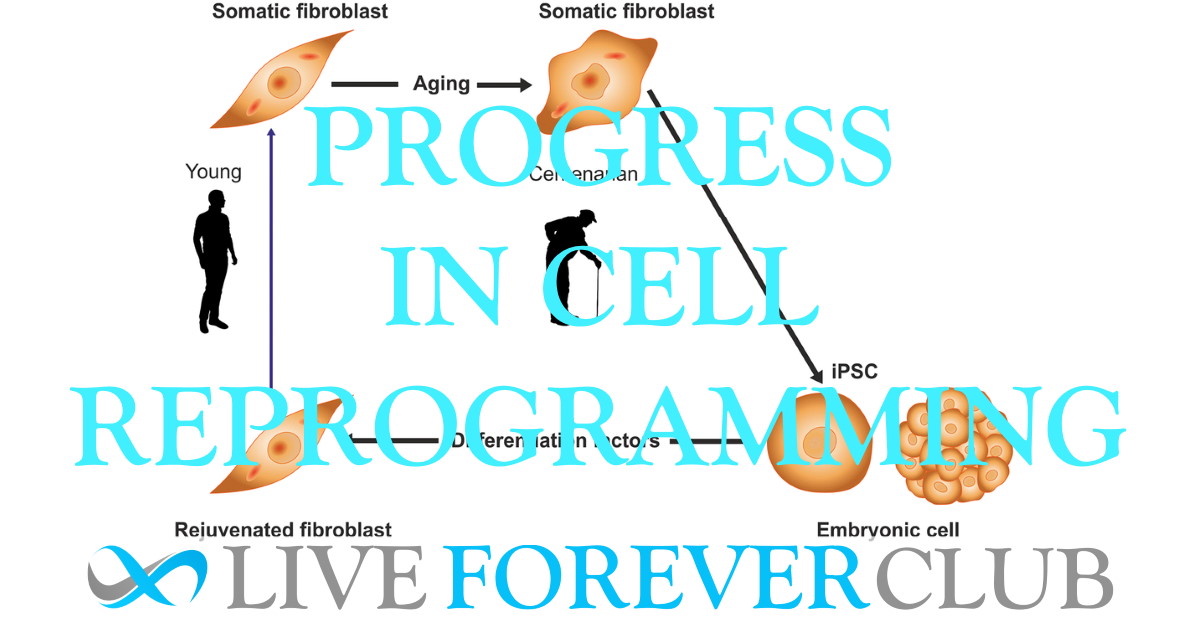
The research was led by Senior Investigator Rodolfo Goya at Institute for Biochemical Research (INIBIOLP), National University of La Plata, Argentina. He believes that somewhere in the genome of somatic cells, there is a viable genetic program that can be set in motion by a small number of master genes; and we have learned how to use it to rejuvenate cells and to a certain extent, animals.
Stephen Horvath's work since 2013 into epigenetic clock has shown that biological age can be determined by looking at DNA methylation (DNAm) profiles in human cells. These DNAm patterns demonstrate homeorrhesis (or moving homeostasis) which means over a short period of time they are stable, but the over longer timescales the patterns drift predictably through development, adulthood and aging.
Depending on which cytosine-guanine dinucleotides (CpG) are tracked, the epigenetic age can determine, with reasonable accuracy, your chronological age or biological age (PhenoAge clock) or mortality risk (GrimAge clock).
Evidence for a link between the epigenetic clock and ageing is already strong with cancer, obesity, Parkinson’s disease and osteoarthritis among the many pathologies that are associated with accelerated epigenetic aging. Also, it has been found that the clock ticks slower in supercentenarians.
The big question asked by the authors of this paper is whether DNAm drives ageing or is it one component in a modular mechanism? They highlight the difficulty in determining whether any intervention to change the rate of biological aging was successful because it manipulated the epigenome or, in practice, it impacted downstream components of the DNA machinery.
The paper then looks at some approaches to rejuvenation by reprogramming cells back to a lower epigenetic age. This makes sense as an approach as the epigenetic clock (nor circadian clock) ticks in embryonic stem cells (ESCs).
It has now been shown that reprogramming somatic cells to induced pluripotent stem cells (iPSCs) sets the epigenetic age back to near zero. In a fascinating experiment in 2011, skin fibroblasts from healthy centenarians were returned to an iPSC state, using a combination of genes (OKSM) before being induced to differentiate back into fibroblasts. The rejuvenated cells (in vitro) behaved just like younger cells with similar mitochondrial metabolism, oxidative stress levels, telomere length and population doubling potential.
Not only is this a remarkable experimental achievement, but it also suggests that the epigenetic mechanisms that control cell function don't deteriorate with age. Good news!
The paper highlights an alternative explanation that cells store a backup copy of their original epigenetic state, though as there is no evolutionary advantage to do this in mammals that aren't able to rejuvenate naturally, the authors regard this as unlikely.
Unfortunately, using OSKM genes to rejuvenate cells in a living body (in vivo) has resulted in the formation of tumours (teratomas) so a slightly different approach is being looked at. Cyclic partial cell reprogramming uses multiple cycles of switching OSKM genes on briefly and then off again. This has seen partial rejuvenation of tissue in the two mice experiments known to have tested this. This partial success opens up the possibility of a modular epigenome, where even if all known age-related methylation points (one module) are reset, other non-clock based sites (another module) may also need to be reset to produce complete rejuvenation.
Other experiments have been carried out with a different set of genes - the Yamanaka factors. These displayed a regenerative ability when injected into living mice (in vivo) using a viral vector (a safe virus delivers the gene cargo). In one experiment, age-related vision impairment was reversed in middle-aged mice, importantly without any pathological side-effects.
The paper also highlights a couple of recent studies where the epigenetic age has been reduced without reprogramming cells. Firstly, Greg Fahy used a combination of human recombinant growth hormone, metformin and DHEA, as a treatment for thymic involution, which resulted in a 2.5 year decrease in relative epigenetic age. And, secondly, in Harold Katcher's famous "elixir" experiment (using a protein fraction from young plasma) the recipient rats saw improvements in the epigenetic age of multiple tissues. Again, there was not full rejuvenation, but on top of the modular options already suggested, when looking at any individual treatment the researchers note that some parts of the epigenome may be insensitive to that a particular approach.
Given the Horvath clock is less than a decade old, it's some achievement that scientists have already shown that forcing it to tick backwards can partially rejuvenate animals. In that short time, we have already discovered two gene cocktails that are able to affect the epigenome – imagine how much we can fine tune their effects over the next decade. And whether or not cyclic partial reprogramming turns out to be a practical anti-ageing therapy, our understanding of the process will be key to this, or any other, cell-level rejuvenation therapy.
Asked by the club about the implication of this research, co-author Jose Cordeiro commented that “It seems that science reality is catching up with science reality. As famous engineer and science fiction author Sir Arthur C. Clarke said: ‘any sufficiently advanced technology is indistinguishable from magic’. Now, it seems that the magic of human technology is making rejuvenation possible.”
Of course, the first cell reprogramming therapies don’t have to work perfectly – perhaps they will only reset half of the age-related methylation sites. But that’s fine, as per Aubrey de Grey’s longevity escape velocity (LEV) approach, that will reduce some of the age-accumulated damage, and by the time you’ve treated other hallmarks of aging (stem cell depletion, extracellular crosslinks, etc.) and return for your next cell reprogramming therapy (say, 10 years later) things will have moved on so that you can reset, again, the original CpG sites, plus more sites (but not necessarily all) that are now accessible with an improved therapy.
Perhaps we could rename the whole longevity escape velocity strategy “cyclic partial rejuvenation” and cyclic partial cell reprogramming is just one component of that?