Key points from article :
DNA changes that are shared by humans and other mammals are linked to life span and other traits.
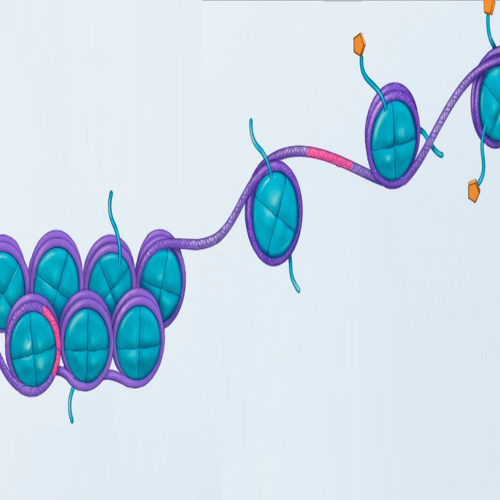
DNA methylation or cytosine methylation can control gene expression by turning genes on or off.
“A highly unique data set unveiled a deeper relationship between DNA methylation, life span, aging, and other biological processes across mammals,” - Jason Ernst, a professor at UCLA.
Methylation differences were focused on DNA sequences that were generally similar.
The Mammalian Methylation Consortium analyzed data from over 15,000 animal tissue samples covering 348 mammalian species.
Intertwined evolution of genome and epigenome found to influence biological characteristics and traits of various mammalian species.
"Long-lived mammals exhibit more pronounced DNA methylation landscapes, while shorter-lived species have more subdued, flatter methylation patterns," - Steve Horvath, senior author.
Findings demonstrate that DNA methylation is subjected to evolutionary pressures and selection.
Using methylation profiles of 185 species, a universal pan-mammalian clock was developed to accurately estimate age in all mammalian species.
The clocks are also predictors of mortality risk in humans and mice, which could be valuable for preclinical studies.
Revealed that developmental genes play a role in the functioning of epigenetic clocks.
Research connects developmental pathways with chronological aging effects and tissue degradation.
Refutes the belief that aging is driven solely by random cellular damage that accumulates over time.
Provides evidence that aging processes are evolutionarily conserved and are closely linked with developmental processes across all mammalian species.
Two studies by UCLA David Geffen School of Medicine and UCLA Health, published in Science and Nature Aging.