Spermidine review – Primeadine tested a for 1 month
What is the impact on physical biomarkers from taking spermidine supplements?
Join the club for FREE to access the whole archive and other member benefits.
In 1678, Anton Von Leeuwenhoek – the father of microbiology – was peering through the lens of one of his self-crafted microscopes at a sample of his own semen, when he spied a strange crystal. Why Leeuwenhoek felt compelled to study this bodily secretion is a tale lost to time, but his curiosity about the crystal led to the discovery of one of the most important anti-ageing molecules in human history – spermidine.
Nearly 200 years later, Phillip Schreiner isolated spermidine along with a related molecule, spermine, from seminal fluid and elucidated their structures. He reported them as ‘polyamines’, which are organic compounds having more than one amine group (composed of a nitrogen atom directly attached to one or more carbon and hydrogen atoms directly, also chemically denoted as NH2). The names of these two molecules, are however, rather misleading as they are not just found in seminal fluid. Spermidine, especially, is abundant in most tissues in the human body as well as many foods such as wheat germ, mushrooms, cheese, soy products, chicken liver, and whole grains.
The true potential of spermidine as an anti-ageing molecule, however, was discovered only towards the second half of the 20th century. Although publications about spermidine and other polyamines can be found as early as 1900, research on these molecules exploded after 1990, once their anti-ageing potential was discovered.
Diets supplemented with spermidine have been found to increase the life spans of worms, flies, and mice. When spermidine is administered as an oral supplement, mice not only live longer, but also have healthier lives. This is because age-associated problems such as disruptions in circadian cycle rhythms, loss of bone density, and development of cardiovascular diseases and neurodegeneration are staved off. In addition, dietary spermidine supplements increase cancer-directed immunity and immunogenic reactions to vaccines along with decreases in fibrosis and carcinogenesis in mice. In essence, spermidine has been proven to have protective effects on the hearts, brains, and immune systems of mice and rats.
In the cardiovascular system, age-induced arterial stiffness was reversed and oxidative damage in endothelial cells was reduced in old mice fed spermidine. In addition, rats genetically prone to hypertension and heart failure when on a high-fat diet, had significantly lower blood pressure levels and arterial plaque formation when fed extra spermidine. This supplementation also had a positive effect on cardiac and skeletal muscle regeneration.
Studies on human populations have shown that human life expectancies are higher in people who have diets high in polyamines, especially spermidine. Several epidemiological studies show that in a diet high in spermidine is negatively associated with development of cardiovascular diseases and death due to heart problems.
Spermidine has neuroprotective effects on cultured nerve cells, and dietary supplementation of spermidine can protect flies against age-related memory impairment and movement problems. In mice genetically prone to encephalomyelitis (an autoimmune condition where the body’s immune system attacks the nerves), spermidine slows the progression of the disease and protects against deteriorating vision. It also promotes regeneration and survival of nerve cells and ganglion cells and can protect against dementia in mouse models of Huntingdon’s disease. In humans, the effect of dietary supplementation of spermidine on memory in older adults is mixed – while some studies show that it has a moderately positive effect on memory enhancement, others show that it has no effects on memory.
Spermidine supplementation can reduce the formation of different types of tumours in mice, and protect against the development of including skin, liver, and colorectal cancers that develop due to age or exposure to toxic chemicals. These effects are thought to be mediated by spermidine’s ability to stimulate immunosurveillance and deplete immunosuppressive cells in the vicinity of tumours. In addition, spermidine has strong anti-inflammatory properties that also inhibit tumour cell growth.
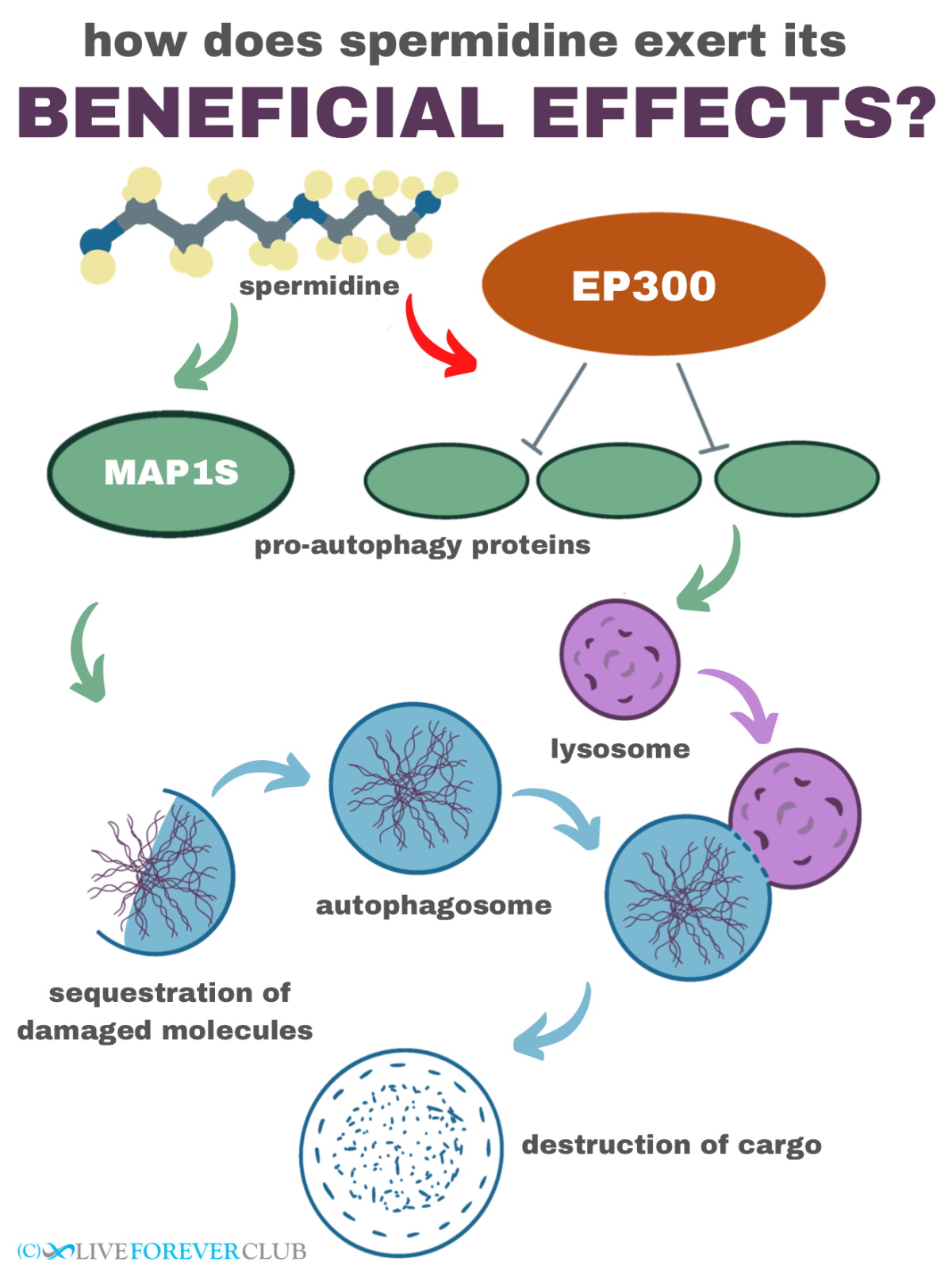
Spermidine exerts its anti-ageing and health-promoting effects via multiple pathways, of which the best studied is autophagy. Autophagy (from the Greek ‘autos’, meaning ‘self’ and ‘phagos’ meaning ‘to eat’) is the process by which cells destroy their old components and recycle them. Autophagy is a self-maintenance mechanism that cells use to literally ‘clean’ themselves by recycling damaged or dysfunctional proteins, protein aggregates, and organelles (such as the energy-generating mitochondria), which could become toxic if not removed.
Spermidine stimulates autophagy to detoxify and reuse potentially harmful cellular components that accumulate in the cells as they age. The autophagy inducing activity of spermidine has been documented in yeast, flies, worms, and mice. Experiments where autophagy is inhibited using drugs or genetic manipulation have shown that without autophagy, the anti-ageing effects of spermidine are lost.
At the molecular level, spermidine inhibits a protein named E1A-associated protein p300 or EP300. EP300 is an acetyltransferase, meaning that it transfers an acetyl group (a molecule made of two carbon, one oxygen, and three hydrogen atoms) on or off other proteins to regulate their activities. Since EP300 acetylates and represses the functioning of several pro-autophagy proteins, inhibiting EP300 activates autophagy. Apart from, EP300 is also involved in the deacetylation of tubulin which reduces autophagy; therefore, EP300 inhibition by spermidine increases tubulin acetylation and promotes autophagy. In addition to its effects on EP300, spermidine also stabilizes the pro-autophagic microtubule-associated protein 1S (MAP1S), and in the long run, affects the gene expression of FoxO3-dependent autophagy network genes.
In mice, spermidine-induced autophagy has been studied in detail in heart muscles, where, a specific type of autophagy known as mitophagy (autophagy of mitochondria) contributes to this molecule’s beneficial effects. In addition to its effects on mitochondrial ‘quality control’, which improve mitochondrial structure and function in heart muscles, spermidine also promotes the production of nitric oxide (NO), a vasodilator that reduces hypertension and suppresses inflammation. Spermidine also helps heart muscles maintain their elasticity and reduce oxidative damage in endothelial cells (the cells that line our arteries and veins).
Through its effects on autophagy, spermidine also promotes immune recognition of cancer cells and counteracts the ability of tumor cells to suppress anti-tumor immune reactions. Apart from these effects, spermidine contributes to strengthening the immune system by boosting immune cell functions and responses to vaccinations. The autophagy inducing effects of spermidine also have broad anti-inflammatory effects by repressing age-associated increases in pro-inflammatory molecules in blood. This not only helps in its anticancer effects, but also inhibits the development of lethal sepsis (a life-threatening condition where an infection can trigger an extreme immune reaction in the body) in ageing mice.
In neurons, spermidine-induced autophagy has two very important effects. One, it helps nerve cells get rid of toxic protein aggregates; and two, it helps nerve cells reset their communications systems by restoring synaptic dynamics. The synapses are points of contact between nerve cells where information is passed on from one neuron to another via bundles of molecules called neurotransmitters. In old flies, memory loss is linked to depressed synaptic function as the cycle of neurotransmitter production, release, and recapture is slowed. By inducing autophagy, spermidine resets this synaptic dynamic and helps neurons reestablish communications systems worn out with age.
In mammals, however, it is unclear if spermidine can cross the blood-brain-barrier on a regular basis. Therefore, the effects of dietary spermidine supplementation in mice and rats may be occurring through systemic changes in the immune system and immune cells that affect the brain. In particular, the anti-inflammatory properties of spermidine are thought to help in suppressing the effects of autoimmune disorders that demyelinate neurons. Demyelination (which occurs in conditions like encephalomyelitis and multiple sclerosis) is the removal of the myelin sheath (an insulating covering on nerve cells that helps them transmit electrical impulses in a fraction of a second) which causes neurological problems. Spermidine prevents the activation of autoimmune-reactive immune cells and alleviates nerve damage.
Although our cells synthesize spermidine from several amino acids, this is not adequate for our bodies’ requirements of this molecule. Therefore, external sources of spermidine – either through ingested food or production by gut bacteria – are important for humans. Normal daily intake of spermidine in humans can vary between 7–25 mg or more. Foods like aged cheese, natto, soy products, and diets like the Mediterranean diet help in maintaining high blood levels of spermidine, which are seen in healthy nonagenarians and centenarians.
However, high dietary intake of spermidine may be contraindicated in some conditions. For example, there is some evidence that high spermidine diets are associated with the risk of developing colorectal adenomas in women. In addition, it is possible that spermidine supplementation can actually support cancer growth (since spermidine promotes cell growth) once cancer has already developed.
In other cases, spermidine supplementation may not be beneficial for people with chronic kidney failure. This is because the breakdown products of spermidine, putrescine and acrolein, are quite toxic if they are not eliminated quickly by urinary excretion. Besides this, some mouse models of Alzheimer’s disease show worsening symptoms when spermidine is added to their diets; this is likely because putrescine (which is a breakdown product of spermidine) encourages neuroinflammatory changes to occur in response to the beta amyloid plaques that develop in the brains in Alzheimer’s disease.
Despite these few contraindications, spermidine is still one of the best anti-ageing molecules discovered till now.
Spermidine is considered to be an excellent anti-ageing supplement as it mimics the positive effects of calorie restriction. Studies on various organisms – yeasts, worms, flies, mice, rats, primates, and even humans – have shown that calorie restriction can not only help organisms live longer but can also improve health. Numerous studies have documented how calorie restricted diets can reduce incidences of cancer, improve heart health, limit neurodegeneration, and strengthen immunity.
However, practicing calorie restriction – which involves special diets and/or intermittent fasting over long periods of time – is often not easily accepted. In addition, the vast changes in eating habits that calorie restriction can require may not be practical for many people. Furthermore, managing such diets to avoid undernourishment, deficiencies, and other unwanted effects is often difficult to achieve.
This is why dietary supplementation with calorie restriction mimics – such as spermidine – are attractive and potentially feasible strategies for healthy ageing regimens. Spermidine is an especially appealing candidate due to its low toxicity yet strong efficacy. This is evident in studies that have found that even lifelong dietary supplementation of spermidine does not seem to have any negative effect on mice. This is why spermidine and its synthetic analogues are highly favoured candidates as dietary promoters of healthy ageing.
Blog written by Anusha Krishnan
Madeo, F., Eisenberg, T., Pietrocola, F. and Kroemer, G., 2018. Spermidine in health and disease. Science, 359(6374), p.eaan2788.
Madeo, F., Carmona-Gutierrez, D., Kepp, O. and Kroemer, G., 2018. Spermidine delays aging in humans. Aging (Albany NY), 10(8), p.2209.
Ni, Y.Q. and Liu, Y.S., 2021. New Insights into the Roles and Mechanisms of Spermidine in Aging and Age-Related Diseases. Aging and disease, 12(8), p.1948.
Wallace, H.M., 2009. The polyamines: past, present and future. Essays in biochemistry, 46, pp.1-10.
Minois, N., 2014. Molecular basis of the ‘anti-aging'effect of spermidine and other natural polyamines-a mini-review. Gerontology, 60(4), pp.319-326.
https://spermidinelife.com/en/blogs/articles/how-spermidine-got-its-name; first accessed on 10th October 2022
Bachrach, U., 2010. The early history of polyamine research. Plant Physiology and Biochemistry, 48(7), pp.490-495.
Wirth, M., Benson, G., Schwarz, C., Köbe, T., Grittner, U., Schmitz, D., Sigrist, S.J., Bohlken, J., Stekovic, S., Madeo, F. and Flöel, A., 2018. The effect of spermidine on memory performance in older adults at risk for dementia: A randomized controlled trial. Cortex, 109, pp.181-188.
Wirth, M., Schwarz, C., Benson, G., Horn, N., Buchert, R., Lange, C., Köbe, T., Hetzer, S., Maglione, M., Michael, E. and Märschenz, S., 2019. Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)—study protocol for a randomized controlled trial. Alzheimer's research & therapy, 11(1), pp.1-17.
New cancer therapies in 2022 achieving unprecedented remissions - reviewing a year of hope
Can love defeat death?
What is the impact on physical biomarkers from taking spermidine supplements?