Ageing is an inevitable biological process marked by a gradual decline in physiological functions, making individuals more susceptible to a range of chronic and degenerative diseases. Among the numerous molecular changes associated with ageing, glycosylation—particularly of Immunoglobulin G (IgG)—has garnered significant attention. IgG glycosylation, a post-translational modification, plays a crucial role in modulating immune responses and maintaining physiological balance.
Alterations in IgG glycosylation have been linked to various ageing-related diseases, offering insights into disease mechanisms and providing potential biomarkers for diagnosis and therapeutic targets.
Overview of IgG Glycosylation
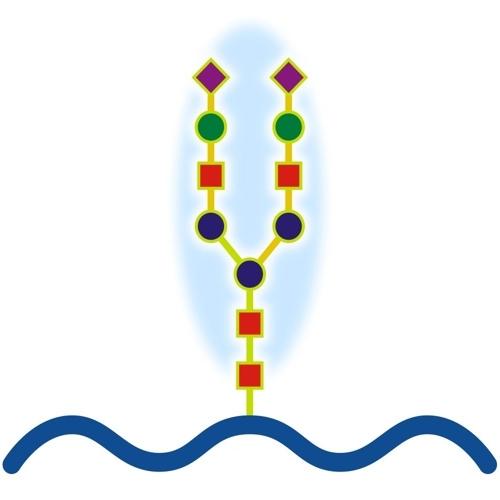
Immunoglobulin G (IgG) is a major glycoprotein in human serum, constituting a significant component of the immune system. IgG molecules are composed of two heavy chains and two light chains, forming a Y-shaped structure with two antigen-binding regions (Fab) and one crystallisable fragment (Fc) region. The Fc region is particularly important for the effector functions of IgG, such as binding to Fc receptors on immune cells and activating the complement system.
Glycosylation is a critical modification of IgG, involving the attachment of carbohydrate molecules (glycans) to specific asparagine residues. This process primarily occurs in the endoplasmic reticulum and Golgi apparatus and significantly influences the biological activity of IgG. N-glycosylation, the most common form of glycosylation in IgG, involves the addition of glycans to the asparagine residues in the Fc region. The structure and composition of these glycans can vary, affecting IgG's ability to interact with other molecules and cells.
The glycan structures on IgG include galactose, sialic acid, fucose, and N-acetylglucosamine, among others. These glycans can be further modified through processes like galactosylation, sialylation, and fucosylation, each of which has distinct effects on IgG function. For instance, galactosylation involves the addition of galactose to the glycan structure, while sialylation adds sialic acid. These modifications are crucial for regulating immune responses, as they influence IgG's interaction with Fc receptors and complement proteins.
IgG Glycosylation and Ageing
As the body ages, the process of glycosylation undergoes significant changes. These alterations are not merely a consequence of ageing but are also actively involved in the ageing process itself. IgG glycosylation patterns change notably with age, leading to modifications in immune function and contributing to the development of age-related diseases.
One of the most well-documented changes in IgG glycosylation with ageing is the reduction in galactosylation and sialylation, coupled with an increase in agalactosylation and bisecting GlcNAc. Galactosylation, as mentioned earlier, involves the addition of galactose to IgG glycans. Studies have shown that the level of galactosylation of IgG decreases progressively with age, with a significant increase in agalactosylated IgG (IgG lacking galactose) observed in older individuals. This reduction in galactosylation is associated with an enhanced pro-inflammatory state, which is a hallmark of ageing.
Sialylation, the addition of sialic acid to glycans, also decreases with age. Sialic acid residues play a crucial role in anti-inflammatory processes, and their reduction can lead to an increased inflammatory response. This change is particularly evident in post-menopausal women, suggesting a link between hormonal changes and IgG glycosylation patterns.
The increase in bisecting GlcNAc, a modification where an N-acetylglucosamine residue is added to the glycan structure, is another age-related change in IgG glycosylation. This modification has been linked to various inflammatory diseases, further highlighting the role of glycosylation in the ageing process.
Alterations in IgG Glycosylation in Ageing-Related Diseases
The alterations in IgG glycosylation observed with ageing are not just biomarkers of biological ageing; they are also implicated in the pathogenesis of several ageing-related diseases. These include neurodegenerative diseases like Alzheimer's disease (AD), cardiovascular diseases, and metabolic disorders such as diabetes.
Neurodegenerative Diseases
In neurodegenerative diseases like Alzheimer's and Parkinson's disease, altered IgG glycosylation patterns have been observed. For instance, in Alzheimer's disease, patients exhibit an increase in fucosylation (the addition of fucose) and a decrease in galactosylation and sialylation of IgG glycans. These changes are associated with the aggregation and deposition of amyloid-beta plaques, a key pathological feature of Alzheimer's disease. The altered glycan structures may impair the clearance of amyloid-beta from the brain, leading to its accumulation and the subsequent neuronal damage observed in Alzheimer's patients.
In Parkinson's disease, similar glycosylation changes have been linked to the misfolding and aggregation of alpha-synuclein, another protein associated with neurodegeneration. The reduction in sialylation observed in Parkinson's patients may enhance the pro-inflammatory properties of IgG, contributing to the neuroinflammatory processes that characterise the disease.
Cardiovascular Diseases
Cardiovascular diseases (CVD), including coronary artery disease and atherosclerosis, are major contributors to morbidity and mortality in ageing populations. IgG glycosylation patterns have been shown to correlate with the risk of cardiovascular events. For instance, increased levels of agalactosylated and bisecting GlcNAc-modified IgG glycans are associated with a higher risk of CVD. These glycosylation changes may promote inflammation and endothelial dysfunction, key processes in the development of atherosclerosis.
Interestingly, the relationship between IgG glycosylation and cardiovascular disease appears to be sex-dependent. Studies have found that certain glycan structures are more strongly associated with cardiovascular risk in men than in women. For example, sialylated glycans without bisecting GlcNAc are negatively associated with CVD risk in women, while the presence of certain fucosylated glycans increases the risk in men.
Metabolic Disorders
Metabolic disorders, such as obesity and diabetes, are also linked to changes in IgG glycosylation. In obesity, alterations in IgG glycosylation are associated with chronic low-grade inflammation, a key feature of metabolic syndrome. Specifically, reduced galactosylation and sialylation of IgG are observed in individuals with higher body mass indices (BMIs), contributing to the pro-inflammatory state seen in obesity.
In diabetes, particularly type 2 diabetes (T2DM), IgG glycosylation changes are linked to insulin resistance and chronic inflammation. Studies have shown that T2DM patients exhibit increased sialylation and decreased bisecting GlcNAc of IgG glycans, which may influence the immune system's response to insulin and exacerbate the inflammatory processes underlying the disease.
IgG Glycosylation in Alzheimer's Disease
Alzheimer's disease (AD) is one of the most studied neurodegenerative diseases, and its connection with IgG glycosylation is particularly compelling. As mentioned earlier, AD patients show distinct alterations in IgG glycosylation patterns, including increased fucosylation and decreased galactosylation and sialylation. These changes in glycosylation are not merely a consequence of the disease but appear to play a role in its progression.
Fucosylation and Amyloid-Beta Accumulation
One of the key findings in AD research is the increased fucosylation of IgG in patients. Fucose is a monosaccharide that, when added to IgG glycans, can alter the molecule's ability to interact with immune cells and other proteins. In AD, the increased fucosylation of IgG is thought to impair the clearance of amyloid-beta, a protein that aggregates to form plaques in the brains of affected individuals. Normally, immune cells like microglia help clear amyloid-beta from the brain. However, fucosylated IgG may alter the immune response, reducing the efficiency of amyloid-beta clearance and thus contributing to its accumulation.
Galactosylation and Inflammation
The decrease in galactosylation observed in AD patients is also significant. Galactosylation is associated with anti-inflammatory properties, and its reduction can lead to a more pro-inflammatory state. In the context of AD, this pro-inflammatory state may exacerbate the neuroinflammation that is a hallmark of the disease. Chronic inflammation in the brain can contribute to the progression of AD by promoting the formation of amyloid plaques and tau tangles, another pathological feature of the disease.
Sialylation and Neuroprotection
Sialylation, particularly the addition of sialic acid residues to IgG glycans, is known for its neuroprotective and anti-inflammatory properties. The reduction in sialylation seen in AD patients suggests a loss of these protective effects, which may lead to increased neuronal damage and cognitive decline. Sialylated IgG is less likely to bind to activating Fc receptors on immune cells, which means it is less likely to promote inflammation. The loss of sialylation thus removes this inhibitory effect, potentially leading to an unchecked inflammatory response in the brain.
Potential for Therapeutic Intervention
Understanding the changes in IgG glycosylation in AD not only provides insights into the disease's pathogenesis but also opens up potential avenues for therapeutic intervention. Modulating IgG glycosylation patterns could be a strategy to enhance the immune system's ability to clear amyloid-beta or reduce neuroinflammation. For instance, increasing the sialylation of IgG could potentially restore its anti-inflammatory properties, while reducing fucosylation might improve amyloid-beta clearance. These approaches are still in the early stages of research, but they represent promising strategies for combating AD.
Conclusion
IgG glycosylation plays a critical role in the immune system, influencing how antibodies interact with other cells and molecules. As the body ages, changes in IgG glycosylation patterns contribute to the development and progression of various ageing-related diseases, including neurodegenerative diseases, cardiovascular diseases, and metabolic disorders. Understanding these glycosylation changes not only provides insights into the mechanisms underlying these diseases but also offers potential biomarkers and therapeutic targets for diagnosis and treatment.
In diseases like Alzheimer's, alterations in IgG glycosylation—such as increased fucosylation and decreased galactosylation and sialylation—are closely linked to the disease's pathogenesis. These changes can impair the immune system's ability to clear harmful proteins like amyloid-beta, promote inflammation, and exacerbate neurodegeneration. By further investigating these glycosylation patterns, researchers hope to develop new strategies for treating or even preventing these devastating diseases.
As our understanding of IgG glycosylation continues to grow, it is becoming increasingly clear that these glycan modifications are not just passive markers of ageing but active players in the ageing process. They represent a critical link between the immune system and the physiological changes that occur with age, offering new insights into how we might better diagnose, treat, and ultimately understand the ageing process and its associated diseases.
The study is published in the journal Acta Biochimica et Biophysica Sinica. It was led by Shisheng Sun from Northwest University.