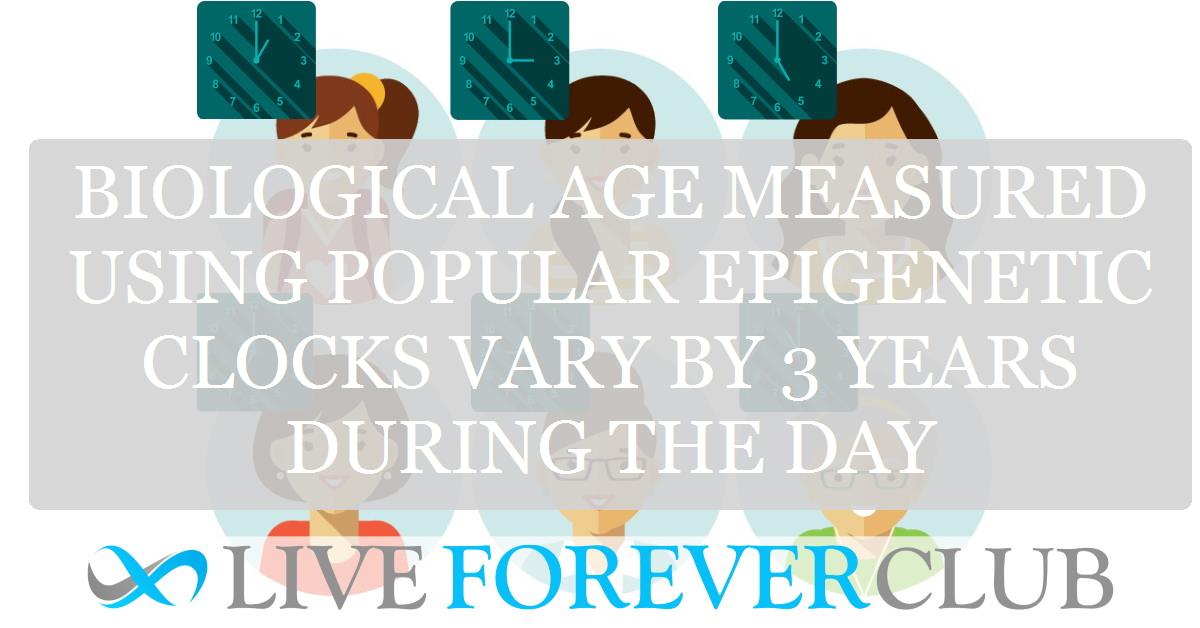Do you ever look in the mirror and wonder where the time went? Why do some people seem to age faster, while others appear to have stopped the clock? Science might be getting closer to answers, and one intriguing tool is called an epigenetic clock.
What are epigenetic clocks?
Epigenetic clocks are tools used in scientific research that measure biological age by examining changes in DNA methylation. Methylation refers to the addition of a methyl group to DNA, primarily affecting the cytosine bases when they are next to a guanine base, known as CpG sites. This modification doesn't alter the DNA sequence itself but does influence gene activity by turning genes on or off, thus affecting how cells function.
As you age, the methylation patterns in your DNA undergo specific and predictable changes. These changes can be mapped and compared to a timeline, allowing researchers to calculate your biological age—a measure of how 'old' your body seems based on the methylation patterns. This biological age can be more telling than your chronological age, which is simply the number of years since you were born.
The difference between chronological and epigenetic age is significant because it can indicate a person's health and predisposition to age-related diseases. For example, a higher epigenetic age compared to one's chronological age can suggest accelerated aging and possibly a higher risk of age-related conditions. Conversely, a younger epigenetic age might imply healthier aging and a lower risk of disease.
Epigenetic clocks leverage this relationship by assessing the methylation status of thousands of sites across your genome. By analyzing these sites, scientists can produce an age estimate that reflects not just the time elapsed since birth but the state of your body's internal biological processes. This advanced method of age estimation has implications for personalized medicine, potentially guiding lifestyle choices and interventions that could mitigate the effects of aging and enhance health span.
Inner clock is more complex than we thought
The perception of epigenetic clocks has fundamentally shifted thanks to new research published in the journal Aging Cell. Previously, scientists believed that epigenetic clocks progressed in a linear, constant manner, ticking forward steadily with age. However, this recent study challenges that assumption, revealing that epigenetic clocks actually oscillate within a 24-hour cycle.
This unexpected discovery indicates that the mechanism of epigenetic aging is far more dynamic than previously thought. The research shows that the epigenetic age of an individual can vary depending on the time of day the DNA methylation is measured. This finding is crucial because it implies that the daily biological rhythms, or circadian cycles, that regulate countless aspects of human physiology, from sleep patterns to hormone levels, also influence the epigenetic markers used to determine biological age.
The oscillatory nature of these clocks suggests that the biological processes associated with aging are interlinked with the body’s circadian rhythms. This integration hints at more complex interactions within our cells that respond to environmental, behavioral, and metabolic signals throughout the day. The study broadens our understanding of how aging might be influenced by daily activities and overall lifestyle, pointing to potential new avenues for research into aging interventions that align with our body's natural rhythms.
Meet your time-traveling cells
The daily fluctuations in epigenetic clocks are driven by the circadian rhythms of your white blood cells (WBCs). These cells are a crucial part of your immune system, tasked with defending the body against both infectious disease and foreign invaders. Intriguingly, various subtypes of white blood cells display different levels of activity at certain times of the day. For example, some types are more active during the day, dealing with the increased metabolic activity, while others peak during the night when the body is resting and repairing itself.
These natural shifts in WBC activity impact the methylation patterns of your DNA. Methylation is a biochemical process that adds a methyl group to the DNA molecule, typically acting to suppress gene expression without altering the sequence itself. As different WBC types rise and fall in prevalence over the day, they each contribute their unique methylation patterns to the overall DNA. This results in daily variations in DNA methylation across your genome, which, in turn, affects the readings given by epigenetic clocks.
Thus, the subtle speeding up or slowing down of your epigenetic age can be linked directly to the rhythmic nature of your immune system's cellular composition. This rhythmic methylation might adjust gene expression patterns in a way that reflects the body’s adaptation to the cyclical demands of metabolic processes, stress response, and tissue repair. Understanding these dynamics offers a deeper insight into how our internal biological clocks correlate with aging and disease susceptibility.
Why this matters for you
This finding highlights a significant challenge for researchers using epigenetic clocks. Since epigenetic age readings can change throughout a single day, scientists need to establish a standardized sampling time. Otherwise, data and conclusions from different studies might be difficult to compare.
Furthermore, this variation could be one factor behind discrepancies between biological age (as measured by the clock) and chronological age (your actual years alive). If an individual's sample happened to be taken when their clock runs 'faster', it could overestimate how biologically aged they are.
This discovery prompts an intriguing line of research: If our body's daily cycles influence our cellular clock, could the reverse be true? Could specific habits—when we eat, sleep, exercise, etc.—alter these DNA methylation patterns?
Understanding the connection between daily routines and the epigenetic clock might lead to lifestyle-based interventions that optimize our biological age. These targeted interventions could support longer healthspan and better well-being overall.
The science of aging is complex. Discoveries like the daily oscillations in our epigenetic clocks prove there's still so much to learn. And while the fountain of youth might still be in the realm of fiction, every study like this gets us a little closer to understanding how we can live longer, healthier, and more fulfilling lives.
The study was carried out by Prof. Dr. Artūras Petronis from Vilnius University and is published in Aging Cell.