Ageing is a complex and multifaceted process, and understanding its underlying mechanisms is crucial for developing treatments for age-related diseases. Osteoarthritis (OA), a degenerative joint disease, is closely linked to ageing, but the exact biological connections have remained elusive. This article delves into research that sheds light on these connections, focusing on the role of DNA damage and repair in the ageing process.
Exploring DNA Damage in Chondrocytes
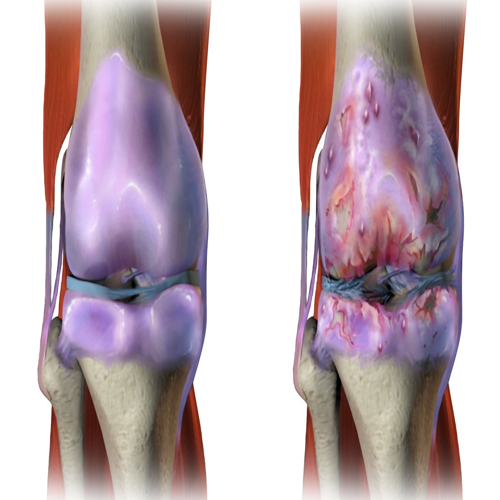
This research conducted a comprehensive analysis of DNA damage in chondrocytes, the cells in cartilage tissue, particularly focusing on age-related changes. It was observed that chondrocytes from older individuals exhibited elevated levels of DNA damage compared to those from younger donors. The study aimed to investigate if this accumulation of DNA damage was due to a decline in DNA repair efficiency with age.
To test this hypothesis, the research used an acute DNA damage model. Chondrocytes were exposed to irradiation, causing immediate DNA damage, and the efficiency of DNA repair was then monitored. The experiment revealed a stark difference in repair efficiency between young, middle-aged, and older donors. Specifically, chondrocytes from older individuals showed a significantly higher percentage of DNA damage post-irradiation and a slower repair rate, indicating a decline in DNA repair capabilities with age.
Furthermore, the study also explored the effects of SIRT6 activation on DNA repair efficiency. SIRT6, a protein known for its role in DNA repair, was activated using MDL-800. This activation significantly improved DNA repair efficiency in chondrocytes from older donors, suggesting that enhancing SIRT6 activity could be a potential strategy to counteract age-related decline in DNA repair and reduce DNA damage in ageing cells.
Sirtuin 6 (SIRT6) to the Rescue
The research on SIRT6 provided an exciting insight into the potential reversal of age-related DNA damage in chondrocytes. SIRT6, a protein involved in DNA repair, becomes less efficient as we age. This study examined the impact of activating SIRT6 using MDL-800, a molecule that enhances SIRT6 activity. The activation of SIRT6 in chondrocytes from older individuals led to a significant improvement in DNA repair efficiency. This was a remarkable finding as it suggested that SIRT6 activation could 'reverse' cellular ageing, at least in terms of DNA integrity.
The study demonstrated that MDL-800, when applied to chondrocytes from older donors, could significantly reduce baseline DNA damage. This was not only true for human chondrocytes but also observed in murine models, highlighting the potential universal application of this approach. The activation of SIRT6 showed promise in reducing the high levels of DNA damage typically seen in aeging cells, thereby mitigating the effects of ageing on cellular function.
Furthermore, the study explored the broader implications of SIRT6 activation. By maintaining lower levels of DNA damage, there's a possibility of preventing the initiation of cellular senescence. This is particularly relevant in the context of diseases like osteoarthritis, where senescence and DNA damage play crucial roles. Thus, SIRT6 activation emerges as a potential therapeutic target to counteract age-related decline in chondrocyte function and possibly in other cell types
The results were clear and promising. Activation of SIRT6 led to a significant reduction in DNA damage, effectively 'reversing' the age of the cells in terms of DNA integrity. These findings offer new insights into how aging affects cellular function and open up potential pathways for treatment.
Beyond the Lab
The implications of the research on SIRT6 and aging extend far beyond the laboratory. This study has the potential to revolutionise our understanding of aging and the treatment of age-related diseases. By demonstrating that the activation of SIRT6 can significantly reduce DNA damage in aging cells, this research opens new avenues for therapeutic interventions in diseases like osteoarthritis. It suggests that we might be able to mitigate or even reverse certain aspects of cellular aging, thereby improving the quality of life for the elderly. Furthermore, this approach could be applicable to other age-related conditions, offering a broad range of potential health benefits. The prospect of targeting cellular aging mechanisms directly could lead to a paradigm shift in how we approach aging and age-related diseases, potentially extending healthy lifespan and reducing the burden of age-related health issues.
Acknowledgments and Credits
This research was conducted at the Thurston Arthritis Research Center, University of North Carolina at Chapel Hill, alongside collaborators from various institutions. Their work, published in the journal AGING, marks a significant step forward in our understanding of ageing and offers hope for new therapeutic approaches to age-related diseases.