Inside every human cell, tiny organelles work quietly to keep life going. Among them, mitochondria are the power generators. They convert nutrients into usable energy and drive almost every cellular process. Without them, our tissues weaken, repair slows, and diseases start to creep in. For years, researchers have explored the idea of transplanting mitochondria to treat illnesses caused by cellular energy failure. But despite its potential, mitotherapy has one major limitation: a lack of reliable, high-quality mitochondria.
A new study from Zhejiang University, published in Bone Research, offers a groundbreaking solution. The research team developed a way to turn stem cells into mitochondria-producing factories. These factories don’t just generate more mitochondria—they produce ones with superior function. In mouse models, these energetic organelles helped repair cartilage damaged by osteoarthritis. This is more than a technical feat. It marks a major step toward scalable, cell-based organelle therapies that could impact millions suffering from metabolic and degenerative diseases.
Why Mitochondria Matter in Medicine
Mitochondria provide over 90% of a cell’s energy. They power everything from muscle contractions to brain activity. When mitochondria break down, the damage ripples across the body. Conditions like neurodegeneration, heart disease, and osteoarthritis all involve mitochondrial dysfunction. In joint diseases, faulty mitochondria leave cartilage cells, or chondrocytes, struggling to survive. They produce less energy, manage stress poorly, and fail to maintain cartilage structure.
Replacing these damaged mitochondria with healthy ones could restore energy balance and slow disease progression. Researchers have experimented with isolating mitochondria from tissues and injecting them into target areas. These early efforts show promise. In heart disease and injury models, mitochondrial transplantation improved recovery. In osteoarthritis models, it helped cartilage regenerate. But the field faces a major obstacle: quantity.
To treat a single patient, doctors may need up to one billion mitochondria per injection. Harvesting that many from donor tissue is invasive, inconsistent, and impractical for widespread use. This bottleneck has limited mitotherapy’s reach—until now.
Turning Stem Cells Into Mitochondria Machines
The research team tackled this problem with a simple question: what if we could grow mitochondria on demand?
They started with mesenchymal stem cells (MSCs), which are known for their self-renewing ability and role in tissue repair. MSCs are easy to obtain, often collected from fat tissue during surgery. But under normal lab conditions, they don’t produce mitochondria in large quantities. The team needed to push them further.
So, they developed a specialized growth medium called the “mito-condition.” This formula included growth signals, vitamins, and nutrients known to support mitochondrial function. Some, like vitamin C and platelet lysate, have already been shown to promote mitochondrial health. After fine-tuning, the team landed on a nine-factor mix that dramatically boosted mitochondrial output.
The results were stunning. Within 15 days, MSCs cultured in the mito-condition produced 854 times more mitochondria than those grown in standard serum. This massive increase was due to both faster cell growth and higher mitochondrial density in each cell.
Quality Matters: These Mitochondria Outperform the Rest
Making more mitochondria is only part of the equation. For therapy, they also need to work better. So the team tested whether their engineered mitochondria actually performed well. They looked at several key markers of mitochondrial health.
First, they measured ATP production—the gold standard for mitochondrial energy output. The mitochondria from mito-conditioned cells produced nearly six times more ATP than those from typical cells. Next, they tested mitochondrial membrane potential, which reflects how well the mitochondria store and transfer energy. These engineered mitochondria held their membrane potential longer, even after being stored.
Reactive oxygen species, or ROS, are another important metric. Excess ROS can damage cells and trigger inflammation. The engineered mitochondria released significantly less ROS, suggesting they operated more efficiently and safely. Even after isolation, they maintained this improved profile.
When these mitochondria were transplanted into other cells, they boosted energy production in the new host. The recipient cells proliferated faster and functioned better, proving these mitochondria retained their potency after transfer.
AMPK: The Molecular Switch Behind the Surge
To understand what was driving this transformation, the researchers turned to transcriptomic analysis. They compared gene expression in mito-conditioned cells versus normal ones. The biggest difference came from activation of the AMPK pathway.
AMPK acts as the cell’s energy sensor. When energy is low, it tells the cell to stop wasteful activities and focus on making more ATP. In mito-conditioned cells, AMPK was fully switched on. This triggered a cascade of changes: higher expression of genes involved in mitochondrial replication, stronger energy metabolism, and faster cell cycles.
One downstream target, TFAM, stood out. TFAM helps maintain mitochondrial DNA and controls how mitochondria are built. The mito-condition significantly boosted TFAM levels. This confirmed that the medium wasn’t just helping cells grow—it was reshaping how they use and produce energy at the organelle level.
But AMPK didn’t work alone. The cell also changed its priorities, reducing other energy-intensive tasks. These included cell migration, secretion, and adhesion. By dialing down those processes, the cell could focus on mitochondria.
A Shift in Cellular Strategy: Energy Over Everything
The mito-conditioned cells were different from normal MSCs in one striking way. They specialized. Instead of performing many functions at once, they focused almost entirely on proliferation and energy production. The researchers found fewer lysosomes and reduced autophagy activity in these cells. While that might sound risky, it made sense in context.
These cells weren’t under stress. Their ER structure remained intact. They weren’t overloaded. Instead, they were operating like a clean energy plant—generating ATP without excess waste or noise. Even organelles like the Golgi and ER remained stable.
This streamlined focus allowed the cells to divide quickly and maintain a high mitochondrial load without burning out. In effect, the researchers had created a new type of cellular state: one that sacrifices versatility in favor of energy efficiency.
For therapeutic use, that’s exactly what you want. You don’t need the cells to do everything. You need them to make mitochondria—and make them well.
Healing Joints with Lab-Grown Mitochondria
The final test was the hardest: could these mitochondria actually repair tissue?
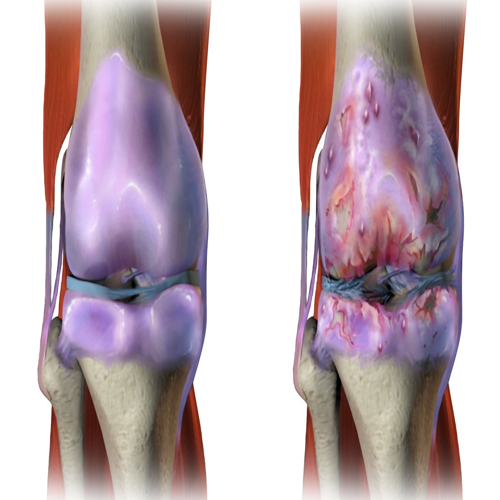
The team injected them into mice with osteoarthritis, a condition where cartilage wears down due to poor cell function and inflammation. The mice received weekly injections of either standard mitochondria or the new high-energy ones. A control group received only buffer solution.
After 12 weeks, the results were clear. Mice treated with the lab-grown mitochondria had smoother joint surfaces, stronger cartilage structure, and higher levels of collagen type II and aggrecan—proteins essential for cartilage health. Bone changes common in osteoarthritis, like spurs and sclerosis, were also reduced.
Even better, producing these mitochondria took less time and fewer cells. The mito-condition reduced the expansion time by nearly 45% and cut the number of starting cells by two-thirds. That efficiency matters. For mitotherapy to scale, production must be fast, consistent, and minimally invasive.
This study showed that lab-made mitochondria could not only match but outperform traditional sources in real disease models.
From Cells to Clinics: What’s Next?
This research lays the groundwork for a new kind of medicine—organelle therapy. Instead of replacing whole cells, doctors might one day repair tissues by restoring their energy engines. And unlike many cell therapies, mitochondria don’t trigger strong immune responses. They offer precision without rejection.
Challenges remain. We still need better delivery methods, especially for targeting mitochondria to the right cells in humans. We need long-term studies to confirm how these mitochondria behave over time. But the foundation is solid.
By reprogramming cells to manufacture organelles instead of tissues, scientists are opening a new chapter in regenerative medicine. The future may not lie in growing new organs. It may lie in rewiring the engines we already have.
And it all starts with a dish of stem cells, carefully tuned to do one job better than nature ever intended: make energy. Lots of it.
The study is published in the journal Bone Research. It was led by researchers from Zhejiang University School of Medicine.