Key points from article :
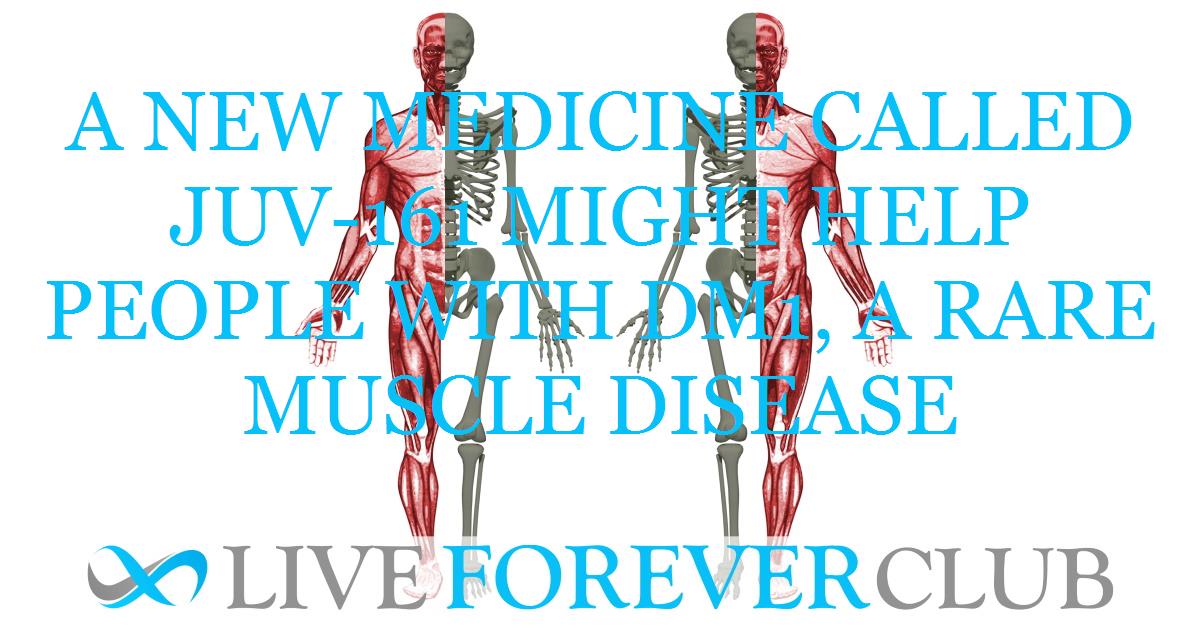
JUV-161, a stem cell protein therapy, received Orphan Drug designation from the FDA for treating Myotonic Dystrophy Type 1 (DM1). DM1 is a rare muscle wasting disease with no current cure.
JUV-161 showed promise in animal models to restore muscle function, improve metabolism, and slow disease progression. Human trials are planned for later this year.
Juvena Therapeutics, the company developing JUV-161, uses AI to identify and engineer stem cell proteins for treating various muscle and metabolic diseases.
This Orphan Drug designation grants Juvena several benefits, including market exclusivity upon approval and tax credits for clinical trials.
DM1 affects about 1 in 2,100 people and causes muscle weakness, cataracts, and heart problems. There is currently no cure.
JUV-161 has the potential to be the first effective treatment for DM1 and represents a significant advancement in stem cell therapy research.