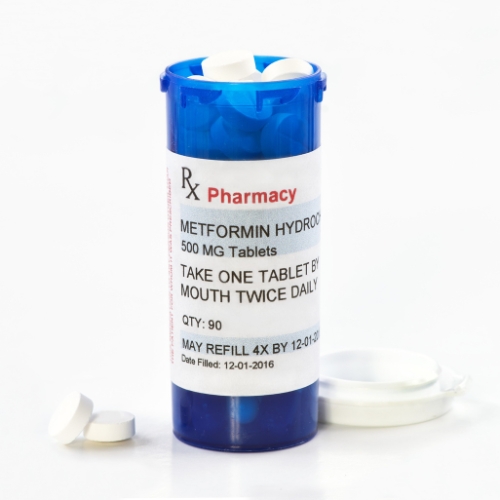
Key points from article :
High levels of a probable carcinogen found in samples of the common diabetes medication.
FDA has has spotted a sixth drugmaker with a contaminated version of the drug.
Bayshore Pharmaceuticals has voluntarily recalled one lot each of its extended-release metformin.
FDA found high levels of probable carcinogen N-nitrosodimethylamine (NDMA) in tested samples.
Lots were manufactured by Beximco Pharmaceuticals in Bangladesh, in June 2019 for U.S. distribution.
Both Bayshore and Beximco say they have not been notified of any side effects.
Last month, Lupin Pharmaceuticals pulled discovered high levels of NDMA in their sample lots.
Also, Virginia-based Granules Pharmaceuticals pulled its own version of extended-release metformin.
Other drugmakers which launched their own recalls include Apotex, Teva, Amneal and Marksans,