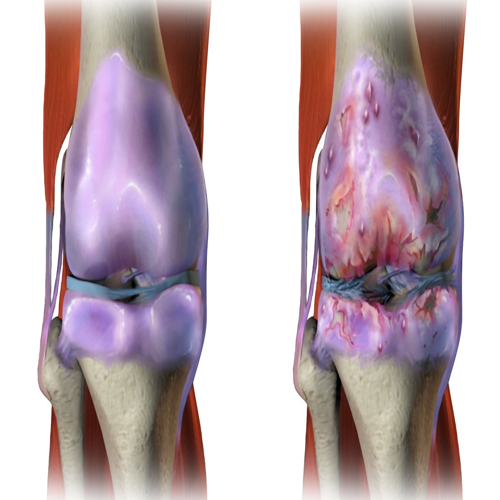
Osteoarthritis (OA) is the world’s most common degenerative joint disorder. It affects hundreds of millions globally, leading to pain, stiffness, and disability. Current treatments often fail to address the root causes of cartilage damage. Standard therapies—such as lubricants and anti-inflammatory injections—offer temporary relief but cannot regenerate cartilage.
A new study, published in The Innovation, introduces a groundbreaking approach inspired by the ancient Trojan Horse. The researchers designed a spatiotemporal drug delivery system that penetrates cartilage, enhances mitochondrial energy production, and restores cartilage health. This article explores the science, the innovation, and the future potential of this technology.
The Problem with Current Osteoarthritis Treatments
Why Osteoarthritis is Hard to Treat
Cartilage lacks direct blood supply, limiting nutrient delivery and drug penetration. The joint environment in OA is harsh:
High oxidative stress due to reactive oxygen species (ROS).
Senescent cell buildup, releasing inflammatory factors.
Excess matrix metalloproteinases (MMPs) that degrade cartilage.
Barriers to drug delivery, such as negatively charged cell membranes and lysosomal degradation.
Because of these hurdles, drugs delivered via injections often remain at the surface and degrade quickly, leaving little impact on deep cartilage layers.
The Need for Precision Delivery
To regenerate cartilage, therapies must:
Penetrate deep into cartilage tissue.
Avoid premature degradation.
Target chondrocytes (cartilage cells) directly.
Deliver drugs in a controlled, sustained manner.
The Trojan Horse Concept
The research team drew inspiration from the Trojan Horse strategy—a deceptive method of penetrating enemy defenses. Instead of soldiers, this Trojan Horse carries fucoidan, a natural marine polysaccharide known for antioxidant and cartilage-regenerating properties.
How the System Works
The engineered construct, called CTNM@FU (Cationic Targeting Nanoparticle-Hydrogel Microsphere@Fucoidan), works in three stages:
Targeting and Transport
Cartilage-targeting peptides guide nanoparticles directly to damaged cartilage.
The positive charge helps them attach to the negatively charged cartilage matrix.
Infiltration and Cellular Entry
Nanoparticles penetrate the cartilage matrix.
They enter chondrocytes while avoiding lysosomal destruction through a “proton sponge effect.”
Controlled Release of Fucoidan
Fucoidan is released in two phases:
Immediate burst release in the first 24 hours to fight oxidative stress.
Sustained release over three weeks to support cartilage regeneration.
This design ensures the right dose is delivered at the right time, directly where it’s needed.
Materials and Methods: The Science Behind the Trojan Horse
Building the Nanoparticles
Mesoporous silica nanoparticles (MSNs) served as the base.
They were modified with amino groups to create a positive charge.
Cartilage-targeting peptides (WYRGRL) were attached for precision binding.
Fucoidan was loaded as the therapeutic cargo.
Hydrogel Microsphere Carrier
Microspheres were created using GelMA (gelatin methacryloyl) and ChSMA (chondroitin sulfate methacryloyl).
These biocompatible hydrogels mimic natural cartilage components.
Microfluidic technology ensured uniform size and porosity.
Together, the nanoparticles and hydrogel formed CTNM@FU, a hybrid delivery system.
Characterization
Electron microscopy showed nanoparticles around 100–250 nm in size.
Charge analysis confirmed the positive surface charge, critical for cartilage penetration.
Spectroscopy validated the presence of fucoidan and peptides.
Results: What the Researchers Found
Deep Cartilage Penetration
In both rats and human cartilage explants, CTNM@FU penetrated deeper than traditional drug carriers.
Retention time was extended to 4 weeks, far longer than standard injections.
Chondrocyte Targeting
CTNM@FU showed strong preference for chondrocytes over other joint cells.
Lysosome escape was successful, preventing premature drug degradation.
Restoring Cartilage Metabolism
Chondrocytes treated with CTNM@FU showed increased collagen type II (COLII) and decreased MMP13, indicating a shift toward cartilage building instead of breakdown.
Gene expression analysis revealed activation of defense, stress-response, and mitochondrial pathways.
Mitochondrial Protection and Energy Boost
Mitochondria are crucial for cartilage health. CTNM@FU treatment:
Increased mitochondrial cristae density.
Enhanced membrane potential and respiration.
Boosted ATP production.
Reduced apoptosis (cell death) and promoted mitophagy (removal of damaged mitochondria).
The Role of SIRT3
SIRT3, a mitochondrial regulator, was found essential for CTNM@FU’s benefits.
Fucoidan bound strongly to SIRT3, activating its protective pathways.
Blocking SIRT3 canceled the positive effects, confirming its key role.
Animal Studies
Post-traumatic OA rat model: CTNM@FU prevented cartilage erosion and restored glycosaminoglycan levels.
Cartilage defect model: CTNM@FU accelerated new cartilage formation, improving collagen fiber orientation and density.
Functional tests (rotarod, gait analysis, pain sensitivity) showed improved joint performance and reduced pain.
Why This Matters
Overcoming the Limitations of Current Therapies
Unlike traditional injections, CTNM@FU delivers drugs directly into chondrocytes, avoids lysosomal traps, and sustains release over weeks.
Targeting Ageing and Senescence
By activating SIRT3, CTNM@FU not only regenerates cartilage but also slows cellular ageing—an important factor since OA worsens with age.
Real-World Potential
Could become a next-generation treatment for OA.
May reduce the need for joint replacement surgeries.
Could be adapted for other degenerative diseases involving mitochondrial dysfunction.
Broader Implications
Regenerative Medicine
Demonstrates how nanotechnology and biomaterials can unlock tissue regeneration.
Precision Drug Delivery
Opens doors for targeted treatments beyond cartilage, such as heart tissue or neural repair.
Future Personalization
With further research, delivery systems may be tailored to disease stage or patient genetics.
Challenges and Next Steps
Safety Concerns
Long-term biocompatibility needs more study.
Effects on non-cartilage cells must be monitored.
Manufacturing Scalability
Producing uniform microspheres and nanoparticles at industrial scale may be complex.
Clinical Translation
Human trials are essential before clinical adoption.
Regulatory approval will require proof of both safety and sustained efficacy.
Conclusion
The Trojan Horse-inspired CTNM@FU system represents a leap forward in osteoarthritis therapy. By combining nanotechnology, biomaterials, and mitochondrial biology, it delivers fucoidan directly into cartilage cells, enhances mitochondrial energy production, and promotes regeneration.
If successful in clinical trials, this innovation could redefine how we treat degenerative joint diseases—transforming OA care from pain management to true regeneration.
The study is published in the journal The Innovation. It was led by researchers from Soochow University.