Key points from article :
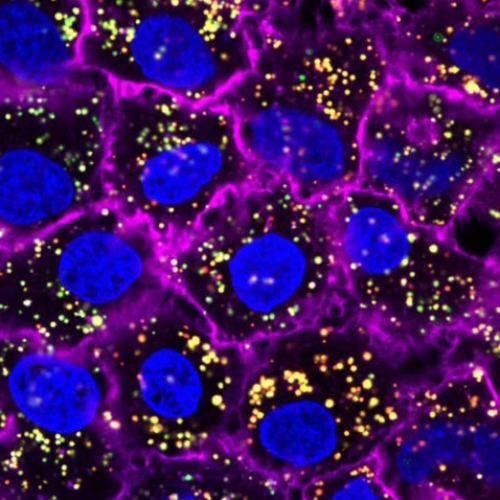
Researchers at Northwestern University have radically redesigned the common chemotherapy drug 5-fluorouracil (5FU), transforming it into a “spherical nucleic acid” (SNA) nanostructure that boosts its cancer-killing power by up to 20,000-fold while reducing toxicity. The study, led by chemist and nanoscience researcher Chad A. Mirkin, reports that weaving 5FU directly into DNA strands wrapped around tiny spheres allows the drug to enter cancer cells more efficiently and target them far more precisely.
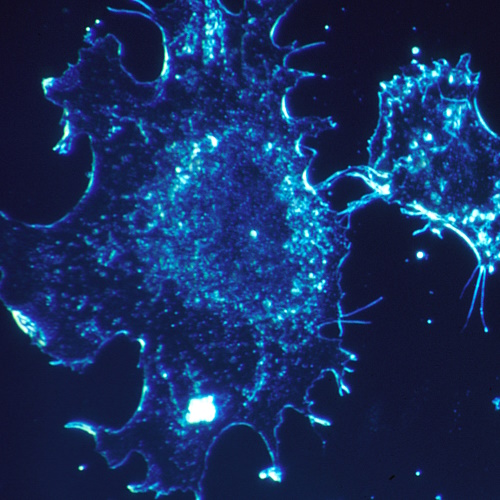
In tests using a mouse model of acute myeloid leukemia (AML)—a fast-growing and difficult-to-treat blood cancer—the SNA-based drug entered leukemia cells 12.5 times more effectively and slowed disease progression by a factor of 59 compared with standard 5FU. Crucially, the treatment left healthy tissues unharmed and caused no detectable side effects, addressing one of the major problems of chemotherapy: its tendency to attack both cancerous and healthy cells.
The breakthrough hinges on how cells naturally absorb SNAs. AML cells have high numbers of scavenger receptors on their surfaces, which recognise and pull in SNA structures without the drug needing to be forced into the cell. Once inside, enzymes break down the DNA coating and release the active 5FU, killing the cancer cell from within. In the mouse experiments, the therapy nearly cleared leukemia cells from the blood and spleen and significantly extended survival.
The team notes that seven SNA-based therapies are already in clinical trials for other conditions, and this new approach could open the door to targeted vaccines and treatments for a range of cancers and immune-related diseases. The researchers now plan to test the therapy in larger animal studies, with the long-term goal of advancing to human trials if results continue to be promising.