Key points from article :
MDI Therapeutics has initiated a Phase I clinical trial to evaluate MDI-2517, a potential treatment for systemic sclerosis (SSc) and interstitial lung disease (ILD). These conditions often occur together, with ILD being a common early complication of SSc. The study’s primary goal is to assess the safety and tolerability of MDI-2517, an experimental drug designed to inhibit plasminogen activator inhibitor 1 (PAI-1), a protein linked to tissue fibrosis and inflammation.
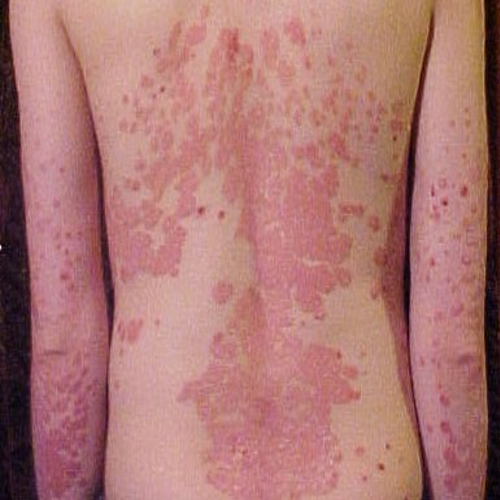
SSc is a rare autoimmune disease that hardens and thickens the skin and internal organs, with a median survival of five to eight years post-diagnosis. ILD encompasses diseases that cause lung scarring (fibrosis), significantly impacting breathing and quality of life. Elevated levels of PAI-1 are associated with both conditions, as excessive activity of the protein disrupts normal wound healing and contributes to fibrosis and inflammation.
Preclinical studies suggest that MDI-2517 can normalize PAI-1 levels, potentially reducing inflammation and fibrosis. During the trial, researchers will also monitor changes in total and active PAI-1 to better understand the drug’s effects on the disease processes. This early-stage trial marks a promising step toward addressing these severe, life-limiting conditions.